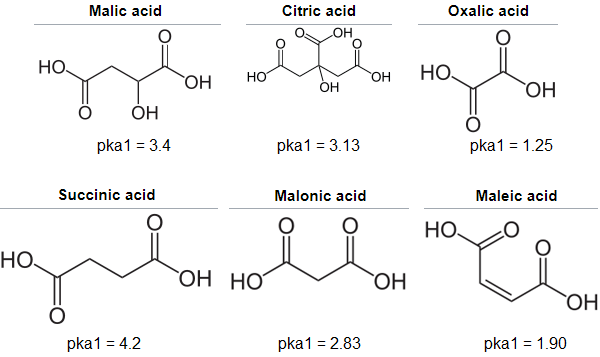
Above is a table of weak organic acids that are produced by plants, which are explained in further detail in the botany page.
Chemicals: the World of Acids, Bases, Oxidizing Agents, and Reducing Agents
Brontsted-Lowry acids/bases are a type of Lewis acids/bases.
The higher the oxidation state of the central atom, the stronger the acid. So covalent oxides (oxides of non-metals) form acids, and ionic oxides (oxides of metals) form bases.
Metal ions behave as acids in aqueous solutions. Generally, the larger the charge and smaller the radius of the ion forms more acidic solutions in hydrolysis reactions (putting ions in water having a pH change).
Acids:
Reacts with Al, Sc, Zn, Fe, Ni, Sn, Cr, Mg, Mn, and Cd to produce hydrogen gas.
Does not react with Cu, Hg, Ag, Au, Pt, Rh, and Ir.
Does not react with glass (except HF does), but reacts with plastic.
However, HNO3 reacts with Cu, but HNO3 is also an oxidizing agent, and it reacts as oxidizing agent instead of an acid. Other metals that do not react with non-oxidizing acids, but react with oxidizing acids, are Si, Nb, Ta, Tc, Re, Hg, Pd, and Ag. However, Ag reacts with HI.
Generally, H2SO4 reacts with the metal listed but does not produce hydrogen gas (such as with Zn), except with Al and Co.
Hot concentrated nitric acid only temporarily reacts Al or Cr, but then an oxide coating is formed, and that oxide coating prevents reacting with nitric acid.
Selenic acid (hot and concentrated) reacts with gold.
Bases:
Reacts with oils/greases and fats.
Does not react with neutral metals, but reacts with metal ions.
Corrodes (dissolves) Si, Sc, Zn, Pb, and Al, also producing H2. These metals are amphoteric metals.
Will dissolve glass bottles, breaking the Si framework, but does not react with plastic.
Does not react with Ge, Ti, Cr, Co, Ni, Zr, Hf, or Cd.
Precipitates with Cu(II) to form hydroxides.
Are stored in polyethylene containers.
Acids dissolve greases, proteins, and carbohydrates.
Bases dissolves greases, proteins, and fats and oils.
Metal cations will react with reducing agents.
The conjugate base of a strong acid and the conjugate acid of a strong base are both neutral, and do not contribute to the pH of a mixture.
Some of the strongest acids in organic chemistry are trichloroacetic acid (pka .64), trifluoroacetic acid (pka .23) and methanesulfonic acid (pka -1.9). The strongest known superacid is fluoroantimonic acid, H2FSbF6.
The only acid exhaled out from the lungs (as a gas) is carbonic acid.
In Lewis acid/base reactions, electrons are transferred, but they are not necessarily transferred in oxidizing and reducing reactions. Anything with an unoccupied low-energy orbital can function as a Lewis acid, and a coordinate bond is formed, and the energy of the lone pair of the Lewis base is lowered because it interacts with the empty orbital of the Lewis acid whose energy is raised. This reaction is reversible, whereas in oxidizing and reducing agents, the electrons are irreversibly transferred.
Bronsted-Lowry acid-base reactions are a type of Lewis acid-base reactions. Lewis acid-base reactions are bond-formation reactions where an atom with a lone pair of electrons forms a new bond with an atom that has an empty orbital, which is different than a 2-electron covalent bond where each atom contributed 1 electron to the bond. Bronsted-Lowry acid-base reactions use this mechanism, as they form a new bond with H+ ion.
In oxidizing and reducing agents, the compound that gets oxidized is a reducing agent and the compound that gets reduced is the oxidizing agent. Iron can be a reducing agent in the Fenton reaction and an oxidizer in the H-W reaction, so it just depends on who wants the electrons more. And in order for the electron transfer to be efficient, the distance must be 14 Angstroms or less (this rule does not always apply to Lewis acids and bases, which can require an even closer distance).
A molecule can be an oxidizing agent under the electron transfer definition, but still be a reducing agent under the loss of oxygen (reduction) or gain of hydrogen definition. Formic acid, for example, a weak acid, is a reducing agent because it donates an electron pair and making the other molecule accept the electrons (so the other molecule is reduced, formic acid itself is oxidized). But the part of it being a weak acid, it is not the acid that is doing the reducing, but its conjugate base formate doing the reducing.
Oxidizing agents can be stored in glass and plastic, but some oxidizing agents, like hydrogen peroxide, cannot be stored in metal or stainless steel. Uric acid is by far the highest concentration anti-oxidant in human blood.
Copper(II) sulfate and Copper(II) nitrate are both an oxidizing agent. Iron(II) sulfate is a reducing agent, while iron(III) nitrate is an oxidizing agent. That is because Fe(II) is reducing, more positive cations are better oxidizers. Cr(II) is a reducing agent while Mn(III) is an oxidizing agent.
Organometallic chemistry.
Metals need to be a good Lewis acid in order to bind to carbon, as well as making carbon a better Lewis base.
Degreasers:
Most degreasers are bases. You can degrease with organic solvents, which are useful to remove oil off of concrete.
Calculating pH:
The formula for calculating pH is not quite pH = -log([H+]), but actually -log(γ[H+]), but γ is usually 1, so it's disregarded. γ = 1 under dilute solutions and low ionic strength, but doesn't equal 1 under higher ionic strength and non-ideal behavior. γ < 1 happens when electrostatic interactions between ions shield them, reducing their reactivity. Higher ionic strength is for lower γ. γ > 1 are rare cases seen in some non-aqeuous solvents or very dilute uncharged species, so hydrophobic environments.
What is HCl at pH 3 = NaOH at pH 11?
pH 11 = pOH 3 = .001 mol/L of NaOH = .01 g NaOH / 250 mL water.
If it were 1 molar, then move the decimal 3 places, which is multiple by 10 3x, so the pH changes 3 units. 1 M of HCl and NaOH is a pH of 0 and 14.
Calculating maximum pH:
What is the pH of 100% hydrochloric acid and 100% sulfuric acid?
In reality, when there is no water, strong acids do not release protons, so there is no pH. But the following are used to calculate the theoretical maximum for strong acids and bases.
This is solved by their molarity at 100%, which is based on their molecular weight. So for HCl and H2SO4, that is -log[12] and -log[2*18] = -1.08 and -1.56. The reason for sulfuric acid being 2 * maximum molarity is due to it being a diprotic acid, having 2 hydrogens.
What is the pH of 100% sodium hydroxide and calcium hydroxide?
NaOH hits a maximum of 4 M at room temperature, and .016 M for Ca(OH)2, which leads to a pH of 14.60 and 12.51. This is calculated from [H+] = 1 * 10-14/(the molarity), then finding the -log() of the value, or pH = 14 - (-log(the molarity)).

Above is a table of weak organic acids that are produced by plants, which are explained in further detail in the botany page.
Amorphous vs. crystalline.
Some compounds like salt are almost always crystalline, while compounds like sodium silicate (Na2SiO3) are almost always amorphous. We will talk about compounds that can exhibit both.
Titanium dioxide used in sunscreen, are generally amorphous, but both amorphous and crystalline can both absorb UV. Crystalline does not absorb UV better despite being more expensive, while amorphous can absorb a slightly wider range of UV. So, this is an example of more expensive is not always better.
Examples of polymorphs:
SiO2.
Amorphous: such as silica gel, used as a dessicant (moisture absorber).
Crystalline: such as quartz, cristobalite, and tridymite.
Al2O3.
Crystalline: such as corundum.
Fe2O3.
Crystalline: such as hematite.
TiO2.
Crystalline: 12 known, such as anatase, rutile, brookite, akaogiite, and riesite.
Anatase and rutile, for example, are tetragonal, while brookite is orthorhombic. Anatase absorbs UV better than rutile and is more reactive, while rutile is more thermodynamically stable than anatase. The electronic bandgap, which determines the energy levels at which electrons can be excited, is generally smaller for anatase than rutile, allowing anatase to absorb higher-energy UV. Star sapphires and rubies get their asterism from oriented inclusions of rutile needles.
TiO2 was found in Dunkin' Donuts powdered sugar donuts before March 2015, after pressure from a public interest group who argued it is not safe for human consumption. The group claims that titanium dioxide is a nanomaterial, which is not regulated or prohibited by the FDA. Titanium dioxide is used to make the powdered sugar appear brighter. Andrew Maynard, director of Risk Science Center at the University of Michigan, rejected the supposed danger from use of titanium dioxide in food, saying titanium dioxide is not a new material or a nanomaterial. Nanoparticles are typically smaller than 100 nanometers in diameter, yet most of the particles in food grade are much larger.
There are also cases where, you want a crystalline center and amorphous surface, which has applications, such as experimental catalysts. An amorphous center with crystalline center, has no known applications.
Sol-gel chemistry.
In sol-gel chemistry, the compounds are irreversible and have no control. But metal-oxide nanocrystal gels can self-assemble a bit, without organic ligands.
Sol-gel chemistry involves hydrolysis and condensation of metal alkoxides or salts, formation of a colloidal suspension (sol), and transformation into a network (gel). Then there is drying or and heat treatment to yield glass, ceramics, or porous materials. A gel is a porous solid phase permeated by liquid. Upon drying or and heat tretment, it becomes a porous xerogel or aerogel.
In sol-gel systems, you want gelation, and not precipitation. Colloids can either precipitate or self-assemble into gels, depending on the conditions. In sol-gel chemistry, you intentionally drive gelation to create porous materials.
Industrial chemistry.
The worst industrial accident in history.
On Dec. 2-3, 1984, in the worst industrial accident in history, more than 3,000 people were killed within hours when toxic gas leaked from a Union Carbide Corporation (UCC) pesticide factory in Bhopal, India. As estimated 14,000 more eventually died, with more than 100,000 suffering injuries. Over 500,000 people in the small towns around the plant were exposed to the highly toxic gas methyl isocyanate. In 1989, UCC paid $470 million (equivalent to $907 million in 2021) to settle litigation stemming from the disaster. In 1994, UCC sold its stake in UCIL to Eveready Industries India Limited (EIIL), which subsequently merged with McLeod Russel (India) Ltd. Eveready ended clean-up on the site in 1998, when it terminated its 99-year lease and turned over control of the site to the state government of Madhya Pradesh. Dow Chemical Company purchased UCC in 2001, 17 years after the disaster.
The UCIL factory was built in 1969 to produce the pesticide Sevin (UCC's brand name for carbaryl) using methyl isocyanate (MIC) as an intermediate. A MIC production plant was added to the UCIL site in 1979. The chemical process employed in the Bhopal plant had methylamine reacting with phosgene to form MIC, which was in turn reacted with 1-naphthol to form the final product, carbaryl. Another manufacturer, Bayer, also used this MIC-intermediate process at the chemical plant once owned by UCC at Institute, West Virginia in the United States.
The worst oil spill in history.
BP, in Gulf of Mexico, had an explosion on April 20 - July 15, 2010, caused 700,000 tons of oil. How many barrels is that? The exact number of barrels in a ton varies with the type of oil, but a good approximation is 7. Each barrel contains 42 gallons. For the BP incident, the U.S. Department of Energy estimated the spill at 4.9 million barrels, or more than 200 million gallons.
Gulf of Mexico had another oil spill at 600,000 tons on June 3, 1979.
Sunscreens.
Has been moved to the newer Cosmetics.html page.
Petroleum, aka crude oil.
It is estimated that the world consumes about almost 100 million barrels (16 million cubic meters) each day (36.5 billion/year), as of 2020. The U.S. consumed 18% of the oil produced in 2015.
Petroleum makes up 40% of total energy consumption in the U.S., but is responsible for only 1% of electricity generation, as of 2017.
In 2018, due in part to developments in hydraulic fracturing and horizontal drilling, the U.S. became the world's largest producer. The top 3 oil-producing countries are the U.S., Russia, and Saudi Arabia (the U.S. was 3rd in 2016).
Transport costs: in the 1950s, shipping costs made up 33% of the price of oil transported from the Persian Gulf to the U.S., but due to the development of supertankers in the 1970s, the cost of shipping dropped to only 5% of the price of Persian oil. Due to the increase in the value of crude oil during the last 30 years, the share of the shipping cost on the final cost of the delivered commodity was less than 3% in 2010.
The U.S. only has 3 major refiners in 2023: ExxonMobil, Chevron, and Phillips 66 (spun off from ConocoPhillips in 2012). Shell and BP have pulled out.
History.
Rare-earth elements.
Rare earths are 17 elements, from lanthanum to lutetium, scandium, and yttrium. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electrical and magnetic properties.
Rare earth elements are important ingredients in electric vehicles, powerful magnets, advanced fighter jets, submarines, smartphones, television screens, automotive and medical industry, microwaves, high strength motors, and many other products. The reason they're called rare earths, is not due to them being rare, but due to how hard it is to separate them from the sources.
China has tremendous power over the rare earths market and supplies nearly 90% of the world’s rare earths because it also is home to most of the processing capacity. In 2017, China produced 81% of the world's rare-earth supply, mostly in inner Mongolia, although it had only 36.7% of reserves. In 2018, Australia was the world's 2nd largest producer, and the only other major producer, with 15% of world production.
The U.S. led the world production from the 1960s up until the mid-1990s, when the Clinton administration closed the Bureau of Mines in 1996. The Bureau's Minerals Information functions were transferred to the U.S. Geological Survey (USGS) in early 1996.
On April 4, 2025, China's Ministry of Commerce imposed export controls of 7 rare-earth elements in response to Trump's tariffs on Chinese products. On May 11, the U.S. and Chinese officials met in Switzerland and reached a 90-day tariff truce.
Organic compounds.
According to a scientist from Argonne Labs in summer 2024, there are ~1060 possible ways to design organic compounds, which is more than all the atoms on Earth (~1050).
Diazoles.
Diazoles are isomeric organic compounds having a 5-membered ring, consisting of 3 carbons and 2 nitrogens. If the 2 nitrogens are next to each other, are called pyrazole, and if they are not, called imidazole. Imidazole is by far, more common. Of the 2 nitrogen atoms in imidazole, the N that is not bonded to H, is basic, whereas the N that is bonded to H, is not basic. Imidazole have high affinity for metal cations. When imidazole is fused to a pyrimidine ring, it forms purine, and purine is the most widely occurring N-containing heterocyclic compound in nature. Imidazole is also in Medetomidine, a synthetic drug developed by Orion Pharma used to put dogs to sleep. In 2023 and 2024, Medetomidine have been found with mixures of fentanyl leading to overdose deaths in the U.S. and Canada.
Imidazole is aromatic, and flat, having 3 pi electron pairs and 2 lone pairs. The nitrogen bonded to the hydrogen has a lone pair in a p orbital, so it's a pi electron pair, totaling 3, making it an odd number of pi electron pairs, making it aromatic. There's 4 p-orbital electrons and 2 p-orbital electrons from the lone pair, making 6, satisfying the 4n +2 rule. (Pi electron pairs are only bonding electrons or lone pairs held in p orbitals.). While both nitrogens are in an sp2 orbital, the nitrogen not bonded to the hydrogen, their lone pair is not part of the aromatic pi-system. That nitrogen uses 2 of its sp2 orbitals to form the C-N sigma bonds, and uses its p orbital to form the double bond with carbon. Hybrid orbitals are not part of a pi bonding system, so that explains lone pairs in hybrid orbitals are also not pi electron pairs.
Part 2: Materials.
Note: there is no standard in English to categorize something from a water molecule, to a smartphone or watermelon. But for this purpose, we simplify these categories as:
Chemicals < materials < objects/appliances.
Among solids, can be divided into ionic and covalent solids, but categories can still overlap. Gemstones, for example, can fall under both. Ruby/sapphire/emerald are ionic solids whereas diamond/quartz/opal/topaz are covalent solids, while garnet and tourmalines are mixed.
Diodes and transistors, resistors and capacitors, can all fall under covalent solids, though some capacitors can have ionic solid components. Diodes and transistors can be categorized as simple semiconductor devices while inverters and rectifiers as more complex semiconductor devices. Transformers are magnetic devices, as well as inductors and electric motors. Operation amplifiers are a type of integrated circuit.
Glass, as of 2008.
The main ingredient in glass is sand (silica). An ancient Egyptian recipe to make glass mixed sand, soda ash (sodium carbonate, lowers melting point), and limestone (calcium carbonate, to improve durability). This chemical mixture was heated to extremes until it became a liquid. This process can still be used today, but there is no single chemical composition which characterizes all glass in our modern world. Thousands of different chemical compositions can be made into glass, and when other substances are added to the glassmaking formula, the final product is affected in various ways. Its color can be changed with metallic oxides: adding iron will make create a strong green or blue-green color, sulfur will provide a yellow to amber hue, silver will give you a pale yellow tint, cobalt deep blue, chromium green, and manganese for purple or decolorizer. Other attributes can also be altered such as the glass’ durability, thermal and reflective properties, and much more. Because of this, glass can be used in a variety of ways and for a variety of purposes.
But it’s not just the ingredients that go into making glass that affect its properties. The way it is made also plays a role—how it is heated, cooled and shaped.
About Glassmaking and Glassblowing
Until about 50 B.C. glass objects could only be made slowly. 1 glass bottle could take several days to make by casting (using a mold), core forming, or cutting techniques. That situation quickly changed with the discovery of glassblowing. Glassblowing was developed in the Syro-Palestinian region around 50–30 BC and spread rapidly through the Roman world. The Roman people discovered that an object could be formed by gathering molten glass on the end of a hollow blowing pipe and inflating it like a bubble.
This technique allows glass to be blown into hollow mold to be formed or freely shaped with simple tools on the end of the blow pipe. As long as the glass remains hot, it can be formed and molded into various shapes, but when hot glass cools, it slowly becomes stiffer and stiffer. Because of that, liquid glass is not like the other liquids; at room temperature, glass is so stiff, it is a very hard and brittle solid.
Ancient glassmakers mastered blue glass early, struggled with red for centuries, and didn't get bright greens until modern chemistry. Green chromium glass was discovered in the 1800s (chromium was discovered in 1797). At 1500 BCE, ancient Egypt and Mesopotamia discovered blue cobalt glassmaking. Purple manganese was discovered by 500 BCE in Egypt and Roman world.
Red glass is the hardest to make. In copper ruby glass, copper compounds are added. The glass must be melted in a reducing atmosphere (low oxygen). Copper ions are reduced to metallic copper nanoparticles, producing red. The challenges are, if too much oxygen, then you get green or blue glass. If wrong temperature, then brown or black, so color depends heavily on furnace conditions.
Although the Romans discovered this red glass technique, the technique was lost after the fall of Rome, and rediscovered in Islamic glassmaking and Renaissance Europe. But using red glass from gold particles was discovered in the 1600s by Johann Kunckel in Germany, and is much harder to control than copper ruby glass.
Elemental metals.
Picture the heaviest naturally occurring element on the periodic table, and you’re looking at osmium. This bluish silver metal is twice as dense as lead, which means a basketball sized sphere of osmium would weigh about 350 to 380 pounds for a volume of 7.0 to 7.5 liters.
Gold resists corrosion better than almost any other element, making it perfect for equipment that faces extreme conditions. Titanium however when exposed to air, immediately forms a very thin, extremely stable oxide layer (TiO2). This oxide layer, is self-healing, blocks further corrosion, and makes titanium appear “inert.” Titanium resists corrosion because it passivates, not because it is inert.
Ruthenium helps make computer hard drives store more data by allowing magnetic layers to be packed more densely together. Annual production worldwide amounts to only about 30 tons, similar to rhodium, with most coming from Russia and South America.
Aircraft engines couldn’t reach their current levels of performance without rhenium. This silver white metal has the 3rd highest melting point of any element, letting it survive the extreme heat inside jet turbines. Engineers blend rhenium with nickel to create superalloys that maintain their strength even when glowing red hot. Only about 50 tons get produced each year globally, mostly as a byproduct of molybdenum mining in Chile and the U.S.
Solar panel manufacturers need tellurium to create thin film photovoltaic cells that convert sunlight into electricity efficiently.
Mexico produces more silver than any other country, followed by Peru and China. Unlike gold, silver tarnishes when exposed to sulfur compounds in the air, creating that familiar dark coating that requires polishing.
Germanium played a crucial role in creating the 1st transistors that launched the computer revolution. Fiber optic cables use germanium oxide to improve signal transmission, carrying internet data across continents at 2/3rd the speed of light. The metal also appears in infrared optics for night vision goggles and thermal imaging cameras that detect heat signatures. China produces about 2/3rd of the world’s germanium supply, extracting it from zinc ores and coal ash. Brazil handles about 90% of the world’s niobium output.
Superconductors.
Metals are not infinitely conductive, as there is some resistance to electron flow. However, in 1911, the Dutch physicist H. Kamerlingh Ohnes discovered that when mercury is cooled below 4.2 K, it loses all resistance to the flow of an electric current. That became known as the superconducting transition temperature (Tc). At warmer temperatures, superconductivity has enormous economic potential. If power lines, or conductors in various electrical devices were capable of conducting current without resistance, enormous amounts of energy could be saved. Superconductors are used in magnets of MRI scanners widely employed for medical imaging. Superconductivity is also associated with perfect diamagnetism, above the Tc.
In 1954, Nb3Sn discovered with Tc of 18 K, then 1973 discovery of Nb3Ge at 22.3 K. Then in 1986, 2 scientists working at IBM, J. G. Bednorz and K. A. Müller, in Zürich, Switzerland, discovered superconductivity at 35 K in a ceramic oxide, LaBa2CuO4, which was the 1st superconductor ceramic. That discovery, which led the 2 to win the Nobel prize in physics in 1987, set off a flurry of research over the world. Before 1987 ended, scientists had discovered YBa2Cu3O7, at Tc of 95 K, then Tl2Ba2Ca2Cu3O10 at 133 K. Because maintaining cool temperatures is costly, liquid nitrogen is the favored coolant because of its low cost (being cheaper than milk), but it can only cool objects down to 77 K.
The physical theory explaining superconductivity is BCS theory, which was published in 1957 and discovered by Bardeen, Cooper, and Schrieffer. BCS theory asserts that the phenomenon of Cooper pairs is the mechanism behind superconductivity.
Cooper pairs: electrons are spin half particles, meaning that a system of 2 coupled electrons behaves as a boson. If we have a group of bosons, then they can all occupy the same quantum state, as opposed to a fermion. Cooper pairs are loosely coupled electrons in a metal, thus these pairs behave as bosons. BCS theory asserts that superconductivity arises as a result of all electrons being coupled as Cooper pairs and that they all occupy the same state. The energy of this state has an energy lower than the Fermi energy.
In 1997, scientists at UIC showed that under pressure, sulfur becomes an elemental superconductor. Years later, experiments with compressed sulfur have shown there is an intermediate state, a bosonic-insulator, become the superconducting properties appear (along low temperature and strong magnetic fields). This means the material changes from a metal, to an insulator, before becoming a superconductor, a novel phenomenon.
10/30/2025.
Scientists have successfully made germanium, a key semiconductor used in computer chips and fiber optics, superconducting for the 1st time. The breakthrough could lead to faster, more energy-efficient electronics and open new paths for quantum technologies. A team of researchers from New York University, the University of Queensland, and other international institutions created a form of germanium that conducts electricity with 0 resistance at 3.5 Kelvin, or about -453 degrees Fahrenheit.
Metal-organic frameworks (MOFs).
Metal-organic frameworks are a coordination network with an open framework containing potential voids. They consist of metal clusters or metal ions coordinated to an organic ligand to form 1-, 2-, or 3-dimenstional structures. A basic formula is organic linker + inorganic node -> MOF (crystalline solids that self-assemble from organic linkers and inorganic voids). Organic linkers are often carboxylic acids, and bases can often knock them off nodes. Inorganic nodes are often metal ions or metal clusters.
MOFs trace back to 1965 when scientists at DuPont synthesized a thermally-stable Zn coordination polymers. With various papers published about them, MOFs started to be made after 2006. In 2021, there are over 100,000 MOF structures registered in the Cambridge Structural Database (CSD), maintained by the Cambridge Crystallographic Data Centre (CCDC). The 1st nanoscale MOF (nMOF) to enter clinical trials on patients is RiMO-301, which is a hafnium-based nMOF which enhances radiotherapy and checkpoint blockade immunotherapy without showing systemic toxicity in preclinical models. As of 2023, there are MOFs that can efficient absorb water vapor, with good selectivty and without also absorbing atmospheric oxygen and nitrogen. There have been attempts to make MOFs to absorb carbon monoxide, but have not been successful.
Most MOFs are not electrically conducting, most MOFs are < 10-9 S/cm, which is barely more conductive than rubber. An example of a MOF that is more electrically conducting is Ni3(2,3,6,7,10,11-hexaiminotriphenylene), at 100 S/cm.
Perovskites.
Perovskites are a class of materials that follow the formula A2+B4+(X2-)3, in which the A cation are generally larger than the B cation, and X is an anion bonded to both cations. That is, compounds that have the same crystal structure as CaTiO3. This mineral was 1st discovered in the Ural Mountains of Russia in 1839 by Gustav Rose, but named after Russian mineralogist Lev Perovski. Since the 2009 discovery of perovskite solar cells, which contain methylammonium lead halide perovskites, there has been considerable research interest into perovskite materials. Many superconducting ceramic materials (the high temperature superconductors) have perovskite-like structures, often with 3 or more metals including copper, and some oxygen positions left vacant. One prime example is yttrium barium copper oxide which can be insulating or superconducting depending on the oxygen content.
Chemical engineers are considering a cobalt-based perovskite material as a replacement for platinum in catalytic converters for diesel vehicles.
Among perovskites, you have 3D and 2D perovskites. 2D perovskites are a newer field of research, which started in 2016. 2D perovskites are more durable than 3D perovskites, which is fascinating.
2D perovskites research are an interest in stable solar cells. 2D perovskites that incorporate diammonium ions instead of monoammonium ions are more rigid and therefore make more stable solar cells. 2D hybrid halide perovskites have ferroelectric behavior. For solar cells, you want a bandgap narrower than 1.4 eV (whereas GaAs is 1.42 at room temperature). 2D perovskites can be as low as 1.1 eV as of early 2025.
A new family of 3D perovskites, called hollow perovskites, which incorporate ethylenediammonium that lead to a massive vacancies of Sn, Pb, and I in the [MX3] framework, are of interest as promising light absorbers with increased air stability that can be utilized in single junction or tandem solar cells.
Halide perovskites.
Halide perovskites follow the formula ABX3, where A = CH3NH3+, CH(NH2)2+, or Cs+. B = Pb2+, Sn2+, or even Ge2+. And X is a halogen. Like fluorophores in organic liquids, their emission Stokes shift increases with temperature (which is a surprise to solid-state physicists). With methyl ammonium for example, they freely rotate in perovskite with dipoles that orient around the charge. Pb halide perovskites are a 1st example of soft anharmonic semiconductors.
Organometallic lead halides, such as CH3NH3PbI3, have revolutionized the solar cell industry with their impressive photon-to-electron conversion efficiency, reaching up to 25%. However, their chemical and structural versatility also enables the growth of crystals ranging from nanometers to 1000 cm³, opening the door to a wide range of intriguing physical phenomena and expanding the scope of potential applications.
MXenes (2D carbides and nitrides).
MXenes were 1st reported in 2011. They follow the formula Mn+1AXn, where M is an early transition metal, A is an element from group 13 or 14, and X is C or N, and n is 1 through 4. MXenes are metals with an electronic state, tunable like semiconductors, and has a variety of plasmonic colors unlike graphene. Ti3C2, for example, is a magnet stronger than aluminum foil. Ti4N3 was the 1st nitride MXene reported. MXenes are better than indium tin oxide (ITO) used in OLEDs.
Alloys used in Apple iPhones.
| 5000 series | 6000 series | 7000 series | |
| Primary alloy | Mg | Mg, Si | Mg, Zn |
| Secondary alloy | Cr, Fe, Mn, Si | Fe, Cu | Fe, Mn, Cu, Ti |
For the iPhone screen starting in the 2016's iPad Pro, and later the iPhone 8 and X in 2017, uses aluminum anodization: an electrolytic process oxidizing the surface to an amorphous Al In 2020, Apple introduced ceramic glass into their iPhone 12 Pro screens (in partnership with Corning), which made it 4x stronger in drop performance than the 11 Pro.
In 2021, Apple introduced gold material in their smartphones, and in 2023, introduced cobalt and copper.
Metal oxide bronzes.
Metal oxide bronzes (not copper-tin alloys) follow the formula AxMOy, where A = H+, Li+, Na+, Cs+, etc., x depends on the level of reduction, M = Mo, W, V, or Nb, and y depends on pre-reduction state of metal. When these bronze layer is connected to an organic layer, they are called hybrid bronzes. These are almost metal-organic frameworks. If there is no porosity, then they are not a MOF, but hybrid bronzes have some porosity. These are a promising set of materials with tunable properties.
Oxidation states.
History - discovey of +9 and +10 oxidation states.
Up until 2010, it was assumed an atom’s oxidation number in a compound could only range between -4 and +8. But an international group of scientists predicted the +9 oxidation state could exist in an iridium oxide cation, [IrO4]+. A team in China confirmed this prediction in 2014, forming [IrO4]+ via the pulsed-laser vaporisation of iridium.
In a recent paper, Wang et al. found an iridium-containing compound with a formal oxidation state of +9. This is the highest oxidation state ever found in a stable compound. To learn if this is the highest chemical oxidation state possible, Kohn–Sham density functional theory was used to study various compounds, including PdO42+, PtO42+, PtO3F22+, PtO4OH+, PtO5, and PtO4SH+, in which the metal has an oxidation state of +10. It was found that PtO42+ has a metastable state that is kinetically stable with a barrier height for decomposition of 31 kcal/mol and a calculated lifetime of 0.9 years. All other compounds studied would readily decompose to lower oxidation states.
Now, graduate student Haoyu Yu (Truhlar group, at University of Minnesota) and Regents Professor Donald Truhlar have a paper published in Angewandte Chemie that reveals that oxidation state +10 exists, in the platinum oxide, PtO42+. This work is featured in the C&EN news and Chemistry World. Using density functional theory, the team looked at the enthalpy profiles and atomic charges of several platinum and palladium compounds. To come to this conclusion, they examined the stability of various transition metal compounds with a metal formal oxidation state of +10 by using Kohn–Sham density functional theory, and found that PtO42+ was stable. PtO42+ has a similar electron density, but a larger partial atomic charge on the metal than one finds in IrO4+, which is the compound with the previously highest oxidation state. Note that PtO42+ and [IrO4]+ are isoelectronic with the commodity chemical OsO4.
PtO42+ is the most stable in the +10 state with a lifetime of 313 days.
Interestingly, the lowest oxidation state in nature was also determined within the Department of Chemistry at the University of Minnesota. Professor John Ellis who reported the oxidation state of -4 for the super-reduced species Na4M(CO)4, where M can be Cr, Mo, or W in their lowest oxidation states of -4 (1983).