Cosmetic Chemistry
As of 2008, the world's largest cosmetic companies are L'Oréal, Procter & Gamble, Unilever, Shiseido, and Estée Lauder.
Shampoos.
The 1st company to include zinc pyrithione in anti-dandruff shampoos is Proctor & Gamble. That was discovered by scientists at Proctor & Gamble in the 1950s, and 1st launchest in 1961, as Head & Shoulders. Adding ZnO to it stabilizes it, which was approved in 1977 as a Japanese patent, and in 1979 as a U.S. patent.
Chelants, like EDTA or and citric acid, are added to shampoos to bind and sequester metal ions such as Ca2+, Mg2+, Fe3+, and Cu2+. Chelants do not bind to keratin, which is the main structural protein in hair fibers. EDTA is ethylenediaminetetraacetic acid.
Skincare.
Niacinamide, which is a form of vitamin B3, is used in skincare such as enhancing ceramide production, help prevent dryness and retain moisture, and managing oily skin and acne. Hindustan Unilever (a subsidiary of Unilever) patented the Fair & Lovely cream in 1971, highlighting niacinamide's role as a melanin supressor, and launched the cream in 1975.
The combining of niacinamide with vitamin C led to a fear of the formation of nicotinic acid, potentially causing skin irritation, in the 1960s. However, these studies involved conditions with high temperatures and prolonged exposure, neither of which are typical in standard skincare usage. This research was finalized in the Journal of Cosmetic Dermatology in 2004.
For example, OLAY super serum uses these 5 main ingredients: activated niacinamide in low pH, a derivative or vitamin C (3-O-ethyl ascorbic acid), collagen peptide (palmitoyl pentapeptide-4), vitamin E (tocopheryl acetate), and alpha hydroxy acid (lactic acid).
Febreeze (Proctor & Gamble).
Hydroxypropyl beta-cyclodextrin, which is ativated by water, was the secret ingredient in Febreeze, was 1st launchest in March 1996, and by June 1998, it was no longer a secret ingredient. The molecules trap and bind volatile odor compounds within its ring structure, effectively reducing their volatility and smell.
Hair straighteners.
2003 is when relaxers used to straighten hair were being replaced withp polymers. Relaxers used alkaline chemicals like NaOH and ammonium thioglycolate. They were being replaced by formaldehyde-releasing polymers, which helped the polymers cross-link.
Sunscreens.
According to multiple industry reports, the leading players in the sunscreen/suncare market are:
-L’Oréal S.A.: Holds over 18% of the global market, the largest share among all companies.
-Beiersdorf AG (Nivea, Eucerin, Coppertone): Around 14 % global market share.
-Johnson & Johnson: Included among the top 3 in sun care products.
-Procter & Gamble (P&G).
The 1st sunscreen, invented in Australia by chemist H.A. Milton Blake in 1932, formulated with the UV filter "salol" (phenyl salicylate) at a concentration of 10%. Its protection was verified by the University of Adelaide. In 1936, L'Oreal released its 1st sunscreen product "Ambre Solaire," formulated by French chemist Eugène Schueller, who founded the company in France. 1978 is when the FDA requires all sunscreens with the SPF rating.
In 1957, Plough acquires Coppertone, then in 1971, Schering Corporation merges with Plough to rename Schering-Plough.
In 2001, Playtex Products bought Banana Boat (which was started by Robert Bell in 1992), but was then bought by Edgewell Personal Care in 2007 (see full details below).
In 2007, Playtex Products bought Hawaiian Tropic for $83 million, but was acquired by Energizer Holdings later that year for $1.16 billion. In 2015, Energizer Holdings spun out several businesses including Playtex Products into a new company called Edgewell Personal Care.
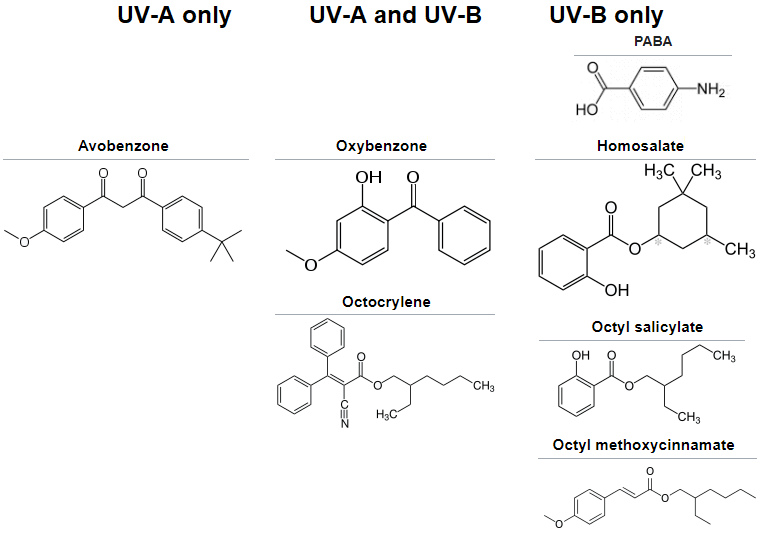
The 1st companies to introduce sunscreens containing zinc oxide or titanium dioxide as active ingredients include Banana Boat and Coppertone, in the 1990s, as micronized versions. This was eventually to replace earlier chemicals such as PABA (Para-Aminobenzoic Acid). Water-resistant sunscreens were introduced in 1977.
PABA (para-aminobenzoic acid)
PABA was 1 of the earlier sunscreen ingredients, before being discontinued. According to a 1982 paper from The Journal of Investigative Dermatology, titled "The Sensitization of Near-Ultraviolet Radiation Killing of Mammalian Cells by the Sunscreen Agent Para-aminobenzoic Acid," it acknowledges that PABA is "1 of the most widely used" ingredient in sunscreens. And it concludes, "In summary, our results with mouse lymphoma cells in vitro clearly demonstrate increased cell inactivation by near-UV radiation when PABA is present. However it must be strongly emphasized that these results may not be taken as evidence that PABA will be photocarcinogenic in human kin in vivo. Nevertheless it is felt that topically applied pharmaceutical and cosmetic preparations, particularly sunscreen agents, should be screened for their effect on induction of damage by near-UV radiation and that in vitro tests may contribute information to a suitable screening protocol."
Oxybenzone
Oxybenzone has been used in sunscreen since the 1980s. In 2021, the U.S. FDA changed their classification of oxybenzone and no longer regard it as GRASE (Generally Recognized As Safe and Effective) due to the lack of data to support its safety despite it being the most common UV petrochemical filter. As of 2019, oxybenzone is used in 70% of sunscreen products, commonly used at 6% in sunscreen (also the max allowed in the U.S.), is a recognized endocrine disrupting chemical (EDC) and is small enough to pass through skin and placenta barrier. According to a 2006 article from ScienceDirect, between octocrylene, octyl methoxycinnamate, and itself, it is the most lipophilic and the only of the 3 found in urine.
Avobenzone
Unlike PABA and oxybenzone, avobenzone does not absorb UV-B (only UV-A), and was approved by the FDA in 1988. However, PABA being effective against UV-B, was not effective against UV-A. Oxybenzone is effective against both.

Note: see the Microbiology.html page, for some sunscreen pigments that are produced by some bacteria.
Sunscreen case study, from 2007 to 2025.
News.
In 2023, a dozen years after the FDA classified formaldehyde as a human carcinogen, the agency tentatively scheduled to unveil a proposal to consider banning the chemical in hair straighteners. However, the government has not acted on this. DMDM hydantoin was the most common formaldehyde-releasing preservative found, showing up in roughly 47% of skincare products and 58% of hair products that contained formaldehyde releasers. Approximately, 20% of lotions, which are frequently used, list these chemicals as ingredients."
9/2/2025.
The European Union is banning certain gel nail polish brands that contain TPO, or trimethylbenzoyl diphenylphosphine oxide.
TPO is the chemical compound that makes gel polish harden under UV lights and gives it a glass-like finish. It is still legal in the U.S.