Many polymers can be semi-crystalline, such as box 4, PVC, phenolic resins (high vs. low cross-linking), polypropylene (PP), HDPE, Nylon, and keyboards. Although candle wax can be considered an amorphous thermoplastic, it is not a polymer.
Polymer Chemistry
With the exception of metals and some inorganic compounds, practically everything else in the world is polymers. Polymers do not boil, so they do not exist in the gas phase. The largest category of synthetic polymers, are polyolefins.
Petroleum is not a polymer, it is an oligomer.
2 types of polymer reactions: step-growth (which includes condensation reactions), and chain-growth (which includes free-radical and ionic reactions). Step-growth has a slow rate of increasing the molecular weight, whereas chain-growth has a fast rate of increasing the molecular weight. In step-growth reactions, water or carbon dioxide are typically a byproduct, and not in chain-growth. In chain growth, free-radical reactions are generally faster than ionic reactions, due to less stabilization.
Step-growth reactions tend to be endothermic, whereas chain-growth reactions tend to be exothermic after the initiator (the initiator is endothermic).
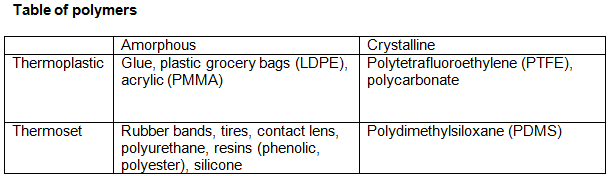
The 2 by 2 types of polymers:
Thermoplastics (amorphous or crystalline), such as glue. They are soft when you heat them. Easier to recycle.
Thermosets (amorphous or crystalline), such as epoxy resins and polyurethanes. Have high cross-links. Difficult to recycle.
Elastomers (amorphous only), such as rubber bands, tires, and contact lens. Are unrecyclable, and have low cross-linking.

Many polymers can be semi-crystalline, such as box 4, PVC, phenolic resins (high vs. low cross-linking), polypropylene (PP), HDPE, Nylon, and keyboards. Although candle wax can be considered an amorphous thermoplastic, it is not a polymer.
Thermoplastics are polymers that can be melted and reshaped via temperature and pressure. Thermosets are polymers that decompose before they can be melted or reshaped.
Elastomers are usually thermosets, but can be thermoplastics. Elastomers are low-cross linked amorphous polymers where the use temperature is above their glass-transition temperature. An adhesive is a linear or branched amorphous polymer that is also used above their glass-transition temperature. Adhesives are typically thermoplastics. Plastics can only be amorphous or partially amorphous.
Viscoelastics are usually an amorphous thermoplastic, but viscoelastic foam can be an elastomer.
Amorphous compounds have no melting point (only glass-transition temperature). Rubber bands are above the glass-transition temperature. Examples of amorphous compounds in inorganic chemistry, is glass. Examples of amorphous compounds in organic chemistry is candle wax. Substances like diboron trioxide can be amorphous or crystalline, depending on the conditions in which it was produced.
Crystalline polymers:
-High melting point.
-High density.
-High resistance to wear and tear.
-Opaque to visible light.
To increase the glass-transition temperature, you can:
-Have shorter ethyl chains, than longer butyl chains. Means less flexible.
-Adding stiffening agents, such as phenyl group side chains such as phenyl rings.
-Adding rigid structures, such as aromatic rings.
-Cross-linking.
To decrease the glass-transition temperature, you can:
-Having longer butyl chains, than shorter ethyl chains. Means more flexible.
-Adding plasticizers.
An example of an important material, which often has large quantities of plasticizers added, is polyvinyl chloride (PVC). PVC without plasticizers has a glass-transition temperature of 81 C. If 30-40% plasticizer is added, the soft-PVC now has a glass-transition temperature of <0 C. Important use of this material are for the manufacture of raincoats, curtains, and cable sheathing.
Table of recycling plastic.
These numbers can be found on the bottom of a plastic container somewhere, where the smaller the number, the easier to recycle.
| 1 | PET or PETE | Polyethylene terephthalate | 2 | HDPE | High-density polyethylene |
| 3 | V | PVC (polyvinyl chloride) |
| 4 | LDPE | Low-density polyethylene |
| 5 | PP | Polypropylene |
| 6 | PS | Polystyrene |
| 7 | - | Other |
The non-recyclables: CD cases are polystyrene (#6), while the discs themselves are polycarbonate, or #7 plastic, which is a catch-all resin number that covers many types of plastic. The casing of tapes is typically polystyrene (#6) (most common), but can sometimes be ABS or other mixed plastics (#7), and audio and video tape is made from polyethylene terephthalate (#1) (typically Mylar).
Car tires are not made from numbered plastics like #1–#7, because they’re not thermoplastics. Instead, they are made from a complex mixture of materials, primarily natural rubber (from rubber trees), and synthetic rubber, especially:
Styrene-butadiene rubber (SBR), most common synthetic component.
Butyl rubber, used for inner liners.
Carbon black, a fine black powder that reinforces rubber and gives it strength and UV resistance.
Steel wires, for structural reinforcement in belts and beads.
Textile fibers, like nylon, polyester, rayon, or aramid (Kevlar) to improve strength and flexibility.
Chemical additives, including sulfur (for vulcanization), zinc oxide, oils, and antioxidants
Car tires are not recyclable through typical curbside plastic recycling because they’re made from vulcanized rubber, which is cross-linked and thermoset, it can’t be melted and reshaped like thermoplastics. Instead, tires are often shredded and used for rubber mulch or asphalt filler, burned as tire-derived fuel (TDF), and reclaimed into products like mats or flooring.
Glue.
Glues are composed of an adhesive agent, additives (such as filling agent, resins, preservatives, or moisturizers), and solvents. There are natural adhesive agents (such as natural rubbers, starch, and casein), but synthetic adhesive agents are more commonly used. Additives are used to improve the adhesive strength and duration of storage life. As binding agents are solids, they are dissolved in solvents in order to become manageable. Most used solvents are acetone, ethyl acetate, methyl acetate, methyl ethyl ketone (MEK), and white spirit. There are also glues which are solvent-free, where for most cases water is then used to dissolve the binding agent and additives.
Some types of glue.
1. Wood glue.
There are 3 main types of wood glue:
-PVA (polyvinyl acetate): traditional wood glue, PVA creates a strong wood-on-wood bond but will not adhere as strongly to metal, plastic or other non-porous materials. Also available in water-resistant formulas, for exterior use. Note that because this is the traditional white glue or school glue, this is often its own category.
-Polyurethane (Gorilla glue): waterproof, this type of wood glue can adhere to wood, stone, ceramic, plastic, and metal.
-Hide glue: Used in cabinetmaking, on chairs and other woodworking projects, these stay in place but can be reactivated by water, making it less preferred than other types of wood glue.
2. Super glue (cyanoacrylate).
Super glue bonds quickly and works with a variety of materials and surfaces, so devices like clamps are typically not needed. Super glues will dry clear and are waterproof and fast-setting. Often the best glue for household projects, they repair broken ceramics as well as industrial uses like automotive assembly. This type of glue can vary in toxicity, so it’s important to check the label before use. They cure through a process called anionic polymerization: the moisture in the air serves as a curing agent for cyanoacrylate glues, triggering the polymerization reaction that forms strong bonds.
3. Epoxy.
Epoxy are a mixture of 2 compounds: a resin and a hardener. They're used in a variety of repairs and home improvement projects, are very durable and can bond materials, even in extreme conditions. They're good for filling gaps in wood. They're available in liquid and paste formulas. Liquids can be easier to use for smaller projects while paste will cover larger areas.
4. A contact adhesive with a solvent base = spray adhesive.
5. Contact cement is a rubber-based adhesive that is effective at joining surfaces that may not work with other types of adhesives. It is 1 of the best glues for nonporous surfaces but will bond almost anything.
They have high-strength bond on wood, metal, glass, plastic and more. Optimized for both interior and exterior applications. Dries in 15 to 20 minutes, excellent water resistance, and resists the effects of heat, weather, grease, oil, and household chemicals.
Note: do not confuse contact cement with rubber cement. Unlike contact cement, rubber cement forms a non-permanent bond with non-porous materials. It can be removed from most hard surfaces with simple friction. Contact cement forms a lasting bond that usually takes heat or solvents to break.
Trivia.
1. Are there any types of glues you should not mix together?
Yes, there are certain types of glues that should not be mixed together as they can react chemically and cause undesirable results. Here are a few examples:
-Cyanoacrylate (super glue) and epoxy: mixing these 2 types of adhesives can generate heat and potentially release toxic fumes.
-Polyurethane and epoxy: mixing these 2 adhesives can result in a weak bond and potential foaming, affecting the adhesive's effectiveness.
-Silicone-based adhesive and other types of adhesives: silicone adhesives can inhibit the curing process of certain adhesives, preventing them from fully bonding or hardening.
-Water-based adhesive and solvent-based adhesive: mixing these 2 types of adhesives can cause clumping, ineffective bonding, or slow curing times.
2. Is the material used for caulking, not a glue?
Correct. The material used for caulking is not technically considered a glue but rather a sealant. Caulk is a flexible material that is used to fill gaps and create a seal between different surfaces, such as around windows, doors, or gaps in tiles.
3. What is the chemical difference between polyurethane glue and polyurethane sealant?
Polyurethane glues typically consist of a polyurethane polymer, commonly derived from the reaction of a polyol and an isocyanate. These glues may also contain additives such as fillers, curing agents, or solvents to enhance their adhesive properties.
Polyurethane sealants often have a similar polyurethane polymer base, but their formulations may include additional components like thickeners, plasticizers, and curing agents to achieve the desired sealing properties.