Mutual Exclusion: Where Something Can Do A and B, but Not A And B at the Same time
Perhaps an easy example in humans is writing something with each hand, but not writing with both hands at the same time.
In criminology, 1 cannot be in a gang, and a known snitch at the same time. In law, 1 cannot legally be a police officer, and a felon at the same time (as with a lot of other offices).
We will explore in different fields.
Genetics.
This does not happen. A person can be left-handed while diabetic while homosexual, while having something as a favorite color, all at the same time.
There are of course, obvious restraints in examples. For example, if 1 were blind, then 1 cannot also be red-green colorblind - having the genes to be red-colorblind is therefore moot.
There are several chromosomal regions where deletions and duplications cause different disorders. A person can have a deletion, or a duplication, but would not be likely to have both.
However, a deletion with a duplication would restore normal copy number and may not have a phenotype. In such situations, the deletion and duplication may cancel each other out, resulting in a chromosomal region with a copy number similar to that of individuals with typical chromosome structure. Depending on the specific genes involved, this restored balance may not result in a noticeable phenotype or clinical features.
Examples of duplication is MECP2 syndrome (caused by duplication of the MECP2 gene on the X chromosome), and Potocki-Lupski Syndrome (caused by duplication on the short arm of chromosome 17).
Deletion of MECP2 causes Rett Syndrome, a different condition, which means both cannot occur simultaneously with MECP2 syndrome. Deletion of chromosome 17 region causes Smith-Magenis Syndrome, which means both cannot occur simultaneously with Potocki-Lupski Syndrome.
Chemistry.
Part 1 - finding chemicals.
This can happen. Some chemicals are magnetic. Some chemicals are phosphorescent.
1. But there aren't any chemicals that are both magnetic and phosphorescent, at the same time.
This does not mean a magnet cannot emit light at all. A magnet can still emit light as a blackbody object, provided it is below its Curie temperature, but still above 500 C. Pure iron's Curie temperature is about 770 C, where it can emit red light a little below that. However, no magnet can emit light at room temperature.
1b. A material cannot be magnetic, and a gas at the same time, as magnets require materials to not be a gas.
1c. Ferromagnetism and superconductivity are almost mutually exclusive.
1d. Equally, metallic conductivity and ferroelectric polarization are almost mutually exclusive.
1e. You can find chemicals that can do alpha radiation and beta radiation, you can find chemicals that can do gamma radiation. But so far 1 hasn't found a chemical that can do gamma radiation, along with either of the other 2.
But this is still on the individual compound. There are some better examples where it isn't about finding chemicals. (You can find humans that are left-handed, you can find humans that are right-handed, as well as find humans that are both-handed.).
Part 2 - mutual exclusion.
2a. Sulfur can bond to H, and Cl, but not to both H and Cl at the same time. (But not as a law, only as an observation). In these cases, H and Cl are quick to form HCl.
Sulfur wants oxidation states of -2, 0, +2, +4, or +6. H-S-H exists, and Cl-S-Cl exists, where S is -2 and +2. But H-S-Cl does not exist, it quickly breaks to form HCl and S.
H-S-S-H exists, and Cl-S-S-Cl exists. But H-S-S-Cl does not exist, as it is not stable as a S(+1) cannot bond to a S(-1).
H2-S-Cl2 does not exist as S is 0 again. But, what about H-S-Cl3 or H3-S-Cl? Sulfur is now -2 and +2 oxidation, which is favored.
Similar to H2-S-Cl2, the H and Cl can quickly break off to form HCl.
2b. Phosphorus can bond to H and Cl, but not to both H and Cl at the same time.
Phosphorus wants oxidation states of -3, +3, and +5. PH3 exists (-3) and PCl3 exists (+3). But no combination of P exists with H and Cl. (For example, PH2Cl = 1, PHCl2 = -1.). But there is a H2-P-P-H2 (diphosphane, liquid) and H-P=P-H (diphosphene).
PHCl4 would have an oxidation state of +3 but again has a stable and volatile elimination product: HCl. It would decompose to PCl3 and HCl. PCl3 is not alkaline.
PH4Cl (P = -3) is referenced in literature, but does not have its own Wikipedia article. So, it be somewhat unstable, but the most stable of all the P complexes bonded to H and Cl. PH4Cl is similar to NH4Cl and an ionic compound. It is the aduct of PH3 and HCl and somewhat stable because PH3 is slightly alkaline and HCl is strongly acidic.
SnH2Cl2 does not exist. The simple reason there would be the following decomposition: SnH2Cl2 -> SnCl2 + H2.
Because the inert pair effect of the s-orbitals stabilizes the oxidation state +2. Same is true for Ge and Pb.
However, silicon can bond with H and Cl at the same time. SiHCl3 (liquid), SiH2Cl2 (gas), and SiH3Cl (trichloro, dichloro, and chlorosilane). Note that the last 1 does not have its own Wikipedia article, only mentioned as precusors in other pages.
3. 2 liquids, with different melting and boilding points, can mix together to form an azeotrope (same boiling point), and a eutectic mixture (same freezing point), but so far 1 has to discover if 2 liquids can form an azeotrope and a eutectic mixture at the same time.
-
As already mentioned in other pages, it is possible for a chemical to be both radioactive and fluorescent at the same time. That's because there are 3 types of radioactivity, but at least 1 type is possible with fluorescence.
Economics.
Examples can be strongly ruled by the law of economics: supply and demand.
For example, in social media, and viewing profiles of girls in gangs, it is rare to find cases where girls in gangs post photos of themselves in bikinis, as well as photos of themselves holding guns (while fully clothed), but both cases happen individually. But the idea of photos of girls holding guns while in bikinis, is seemingly something you would have to offer to buy. If you want to throw in a 3rd variable, that is pregnancy. Most photos of pregnant girls can show belly photos, but photos of pregnant girls in bikinis, with their belly exposed, and holding guns, would have a higher monetary demand to buy.
Law.
An example that happened in Chicago, that was fought since at least 1993, was businesses are allowed to serve alcohol, and to allow toplessness performers, but business are not allowed to do both at the same time.
A more common example is restaurants not being allowed to allow cigarette smoking, and serving children at the same time.
For more criminal cases: driving can be legal, using your phone is legal, but not doing both at the same time.
Anatomy.
Trade-offs in humans.
Humans that can see in darkness real well, tend to see less intensity of color, whereas humans that can see color real well, see in darkness poorly.
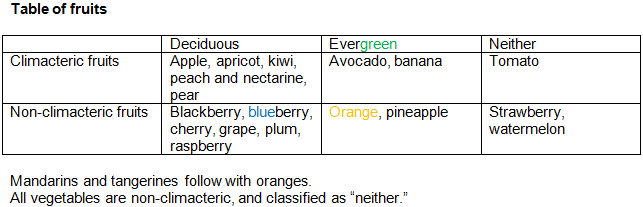
Botany - fruits.

From the above table, fruits occupy 6 cells, whereas vegetables only 1. So something cannot be a vegetable and climacteric at the same time, etc.