Microbiology
The bacteria population globally is 5 * 1030, with viruses at least 10 times more. There are an estimated 100 million types. There are up to 10 billion viruses per 1 liter of sea water, and 1 g of soil. The whole oceans contain 4 * 1030 viruses, which if laid side-by-side, spans 10 million light-years. A drop of seawater contains 500,000 bacteria and tens of millions of viruses. The gut contains a hundred trillion bacteria. Other than sea water and entrances to sea water, bacteria outnumber viruses. There are typically 40 million bacterial cells in a gram of soil and a million bacterial cells in a milliliter of fresh water.
Killing bacteria and viruses: chemicals.
The best (chemical) way to kill viruses and bacteria are oxidizing agents, then acids and bases at a tie. (Some acids kill viruses better, some bases kill viruses better.). Bleach and hydrogen peroxide generally kill Gram-positive bacteria better than Gram-negative. Alcohols such as 70% isopropanol and 70% ethanol can kill viruses and bacteria also. However, 70% isopropanol and 70% ethanol are not effective against killing non-enveloped viruses, but are against enveloped viruses. Ethanol can be bought at 70% concentration, and isopropyl alcohol (isopropanol) can be bought at 70% and 91% concentrations, and 99% concentration on-line. But note that alcohol in this context are not good at killing viruses/bacteria in water, they are better at killing viruses/bacteria on surface. Ethanol is more dehydrating, whereas isopropanol evaporates more quickly.
Another way to kill viruses is UV light. UV radiation kills viruses by chemically modifying their genetic material, DNA and RNA. The most effective wavelength for inactivation is 260 nm, which falls in the UV-C range. The ground-level virucidal solar UV wavelengths fall above 290 nm, which is UV-B range (290 to 320 nm), (UV-A range (320 to 380 nm)). Nucleic acids are damaged also by UV-B and UV-A but with lower efficiency than by UV-C radiation. (For comparison, the human eye is most damaged by UV-C at 265-275 nm, but UV-B from 280-310 nm can cause photokeratis (snow blindness), and can damage the cornea, lens, and retina.).
For air sanitizers, there is triethylene glycol and propylene glycol (both at 4.4%). They disrupt the bacterial cell membrane, causing the cells to leak and lose their integrity.
For killing bacteria in water, there is tetraglycine hydroperiodide (at 16.7%), after waiting about 35 minutes. For a 2-pack, there is a 1st part of 2% chlorine dioxide, and a 2nd part of 5% phosphoric acid, to remove the chlorine.
For killing bacteria in the human eye surface, there is erythromycin, which you squirt as a gel (do not squirt to the cornea of the eye, but the white part of the eye). It is still better at killing Gram-positive bacteria than Gram-negative. Erythromycin binds to the bacterial ribosome, specifically to the 50S subunit, and inhibits bacterial protein synthesis. This can take a few hours to several days. Erythromycin can also be bought at pet stores as tablets for killing bacteria in fish tanks, when fish have bacterial infections.
Sodium azide and phenylethyl alcohol (PEA) is usually good a inhibiting Gram-negative growth, and not Gram-positive.
Note: for anti-biotics and anti-viral drugs, are covered in the pharmacology page.
The majority of bacteria in the world are good bacteria (~90%), with the rest, bad bacteria.
Some non-chemical ways to kill bacteria is heating them, and degassing them. Bacteria generally do not have cardinal temperature ranges of more than 40 C. E. coli, for example, has the most strains ranging from 8 C to 48 C, with 39 C being the optimum temperature (46 F to 118 F). Degassing bacteria is to make it difficult for them to grow.
There are bacteria that kill other bacteria, viruses that kill other viruses, viruses that kill bacteria, but bacteria that kill viruses are not known.
Bacteria trivia.
Bacteria used in sewage treatment tend to be facultative bacteria, that can live in acidic and methane environments, with a mix of Gram-positive and Gram-negative.
Deinococcus radiodurans is an extremophilic bacterium, one of the most radiation-resistant organisms known. It can survive cold, dehydration, vacuum, and acid, and is therefore known as a polyextremophile and has been listed as the world's toughest bacterium in a Guinness Book of World Records before.
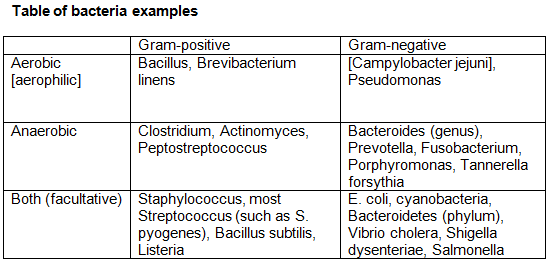
Bacteroidetes (Gram-negative, aerobic or anaerobic, phylum) and Gram-positive bacteria make up 99% of all the bacteria in the large intestine.
The bacteria found in feet and causes foot odor (as well as the rest of human skin), is Brevibacterium linens. It is Gram-positive, rod-shaped, and aerobic.
Bacterias that naturally produce vitamin B12 include Propionibacterium and Pseudomonas, especially Propionibacterium freudenreichii.
Vibrio vulnificus (Gram-negative, rod-shaped) causes more than 95% of seafood-related deaths, commonly found in raw oysters.
Unlike most Gram-negative bacteria, Borrelia burgdorferi cannot recycle its peptidoglycan during growth, leading to the continuous release of unique and inflammatory cell-wall fragments.
Pasteurization.
Pasteurization became a regular method of food safety in the U.S. back in the 1920s. According to the Virginia Department of Health in 2023, most milk is pasteurized by either heating it to a minimum of 145 degrees Fahrenheit for at least 30 minutes (63 C), or to a minimum of 161 degrees Fahrenheit for at least 15 seconds (72 C). This process kills off the bacteria, and then the milk is chilled again.
Endospores, endotoxins, and exotoxins.
The most common bacteria that produce endospores are from 2 gram-positive bacteria: the aerobic Bacillus and the anaerobic Clostridium. Endospores allow bacteria to remain dormant for extended periods, such as centuries. The endospores consist of the bacteria’s DNA, ribosomes, and large amounts of dipicolinic acid (up to 10% of the endospore’s dry weight).
Endotoxins are lipid A, (a piece of outer membrane lipopolysaccharide (LPS) of gram-negative bacteria). Endotoxins that are pathogenic to humans have only been confirmed in Gram-negative bacteria. Endotoxins are not a protein secreted from cells, they only shed off.
Exotoxins are proteins released by gram-positive and gram-negative bacteria. Most gram-positive bacteria release exotoxins. However, examples of gram-negative bacteria that excrete exotoxins are Vibrio cholera, E. coli, Campylobacter jejuni, and Shigela dysenteriae. Examples of bacterial diseases caused by exotoxins are anthrax, botulism, tetanus, and cholera.
Bacterial microcompartments.
Bacterial microcompartments are organelle-like structures found in bacteria made entirely of proteins, and consist of a protein-shell that encloses enzymes and other proteins. They are typically 40 to 200 nm in diameter.
Carboxysomes are carbon-fixing bacterial microcompartments found in photosynthetic bacteria, and are coated by the protein McdB. They consist of shells filled with the enzyme RuBisCO.
Biofilms.
Bacteria can form multi-cellular communities, or biofilms, in which individual cells are protected from environmental attacks (such as antibiotics) by virtue of being (1) encased in a protective matrix comprised of polysaccharides and other macromolecules and (2) physiologically distinct from free-living, planktonic cells. Furthermore, biofilm formation enhances the ability of bacteria to colonize surfaces, including host tissues and abiotic surfaces such as medical implants. As a result of these characteristics, bacteria in biofilms are believed to be responsible for the majority of hospital-acquired infections.
Biofilms are thought to develop on contact lens, which explains why contact lens solution eventually becomes ineffective. PABA, or para-aminobenzoic acid, are inhibitory to biofilm formation, whereas the adhesive protein LapV, is important for biofilm formation.

The bacteria found in urine are typically facultative anaerobic Gram-negative, rod and cocci.
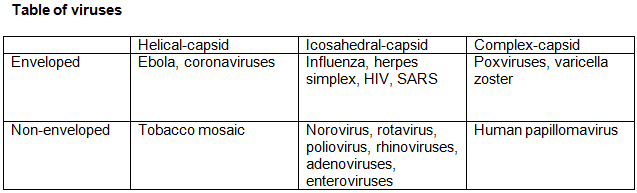
Enveloped viruses have lipid membrane surrounding their capsid, whereas non-enveloped have protein-capsid.
Another class of viruses, bacteriophages, can be enveloped or non-enveloped, depending on the order. For example, Caudoviricetes, known as tailed bacteriophages, which includes the myovirus, are non-enveloped. Bacteriophages are among the most populated viruses, found wherever bacteria exists. It is estimated that there are more than 1031 bacteriophages on the planet, more than any other organism combined. Up to 70% of marine bacteria may be infected by bacteriophages.
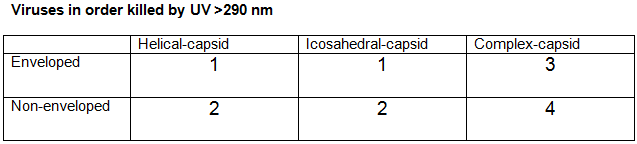
UV light is more sensitive to enveloped viruses, due to the lipid membrane, than non-enveloped viruses. However, UV light >290 nm is also more effective against non-enveloped viruses with simpler structure (icosahedral and helical capsids), than enveloped viruses with more complex structures.
RNA viruses (such as influenza and HIV) generally have higher mutation rates compared to DNA viruses. This is because the replication of RNA is more error-prone due to the lack of proofreading mechanisms during replication, and this allows RNA viruses to evolve quicker.
Covid fatalities:
Accordng to the CDC, Covid-19 ranked 3rd most common cause of death in the U.S. in 2020, 2021, 4th in 2022, and 10th in 2023.
Viruses and bacteria in the stomach:
The most viruses in the human stomach are bacteriophages (97.7%), eukaryotic viruses (2.1%), and archael viruses (.1 %). Yes, bacteriophages are viruses.
The bacteria in the stomach are majority Bacillota (formerly called Firmicutes) and Bacteroidota (formerly called Bacteroidetes). Examples of Bacillota in the stomach are Lactobillus and Clostridium, and examples of Bacteroidota in the stomach are Bacteroides, which are more common in the intestines than stomach.
Viruses and bacteria on the human skin:
It was known in the 2010s that certain viruses found on the skin, such as HPVs and polyomaviruses, activate CD8+ T cells, which fights to prevent cancer, in that they protect against tumor formation. This became a stronger confirmed discovery in 2018. We call them commsensal viruses, as they live in or on a host, without causing harm. CD8+ T cells express the CD8 glycoprotein on their surface.
Some bacteria on our skin, such as Staphylococcus epidermis and aureus, can produce adenosine analogs which can interfere with host cellular processes, including DNA replication. A 2018 study published in Science found that Staphylococcus epidermis also promotes CD8+ T cells responses against skin pathogens and possibly tumors. Some of their strains produce N-formyl peptides, and other molecules that activate antigen-presenting cells lke dendritic cells. These antigen-presenting cells then present antigens to CD8+ cells, triggering their activation.
The immune system of bacteria.
Bacteria have an immune system that stops bacterial viruses (phages). The 1st detection of an immune system in bacteria happened in 1952, where it was called restriction-modification. Not much happened until 2007, with the discovery of CRISPR. Then in 2018, 9 anti-phage systems were discovered, and as of late 2023, there are more than 100 anti-phage systems are discovered. The host-pathogen interactions illuminate the fundamental biology.
Here is an overview process comparing bacteria with mammals.
Mammals: viral infection -> cGAS -> 2',3'-cGAMP -> STING -> Type I interferon.
Bacteria: phage infection -> CD-NTase -> cyclic dinucleotides -> Effector -> programmed cell death.'
cGAS is a human protein, 2'3'-cGAMP is a very small RNA molecule, and it can program cell death (called apoptosis). Cl- efflux can lead to abortive infection (called restriction of phage infection). STING and Cap14 can both encode transmembrane domains, but the 2 are not evolutionarily or structurally related, as STING can be found in organisms without interferons.
Against other bacteria.
An example of a bacteria that kills other bacteria, is Bdellovibrio bacteriovorus. It is Gram-negative, and considered as predatory bacterium. It replicates within the periplasm. It can destroy E. coli. But a way for E. coli to protect against Bdellovibrio is curli fibers. Bdellovibrio is not really known to attack their own species, so there are selectivity theorys.
Against chemicals.
Bacteria such as Vibrio fischeri has a pretty powerful catalase activity that protects it from hydrogen peroxide, and professors have generated a catalase mutant that was much more sensitive.
-
VOCs produced by bacteria.
VOCs are not just produced by plants. An example of a common sesquiterpine is geosmin, a volatile compound produced by bacteria, mainly cyanobacteria, that gives the smell of a rainy day, that are present in soils and water supplies. Petrichor, is the Earthly scent produced when rain falls on dry soil.
Chemical sunscreen produced by bacteria.
Did you know there are compounds that function as sunscreen, that are made by bacteria? 2 of them are scytonemin and nostodione A, which are pigments found exclusively in cyanobacteria. Scytonemin was discovered in 1849 by Swiss botanist Carl Nägeli, and was mentioned in several papers during the 20th century. The compound was not isolated until 1991 when Garcia-Pichel and Castenholz did a in-depth study of it. They identified it in over 30 species of cyanobacteria, where it mainly occurred in the extracellular matrix, which consists mostly of high-molecular weight polysaccharides, but also different proteins. Scytonemin absorbs strongest in the UV-A region (315-400 nm) and UV-C regions (100-280 nm), while less pronounced in the UV-B region (280-315 nm). Scytonemin was also demonstrated to exist in 2 different forms, a predominant oxidized green-brown form and a reduced red form. The 2 could be interconverted by a reducing or oxidizing agents. The unique dimeric structure of scytonemin was determined in 1993 along with its reduced form. Cyanobacteria containing scytonemin were more resilient toward UV-light than those without. Further, scytonemin has been proposed to have an ancient origin, being present as a cyanobacterial sun protective metabolite already during the Precambrian time period (~2 × 109 years ago).
Nostodione A, having a close structural resemblance to scytonemin, has received far less attention. It's been proposed as a biosynthetic precursor in the synthesis of scytonemin. It was 1st isolated from cyanobacteria in 1994 by Kobayashi who characterized it as a pair of rapidly interconverting stereoisomers. This was however not the 1st report of nostodione A. Unknowingly, a semi-total synthesis of the natural product was performed in 1993 when ozonolysis of reduced scytonemin yielded nostodione A during the scytonemin characterization campaign. As of 2014, 2 additional reports on the isolation of nostodione A have since then been published.
Selectivity in detecting bacteria:
The catalase test: catalase is produced by bacteria that respire using oxygen, and protects them from the toxic by-products of oxygen metabolism, and gives bacteria resistance to hydrogen peroxide. The catalase test (by using 3% hydrogen peroxide) is used to differentiate catalase-positive (like Staphylococci) from catalase-negative (like Streptococci). If positive, there is bubbling (hydrogen peroxide being broken into water and oxygen). Catalase-positive bacteria include strict aerobes as well as facultative anaerobes, although they all have the ability to respire using oxygen as a terminal electron acceptor. Catalase-negative bacteria are either anaerobes, or facultative anaerobes that only ferment and do not respire using oxygen as a terminal electron acceptor.
The coagulase test: coagulase is an enzyme produced by Staphylococcus aureus that converts (soluble) fibrinogen in plasma to (insoluble) fibrin. So this is tested with rabbit plasma. Not all staphylococci produce coagulase, so this test can distinguish Staphylococcus aureus from other Staphylococci. Clumping (coagulation) is for positive cases.
The oxidase test: identify bacteria that produce cytochrome c oxidase, an enzyme of the bacterial electron transport chain. All bacteria that are oxidase-positive (such Neisseria and Pseudomonas) are aerobic, and can use oxygen as a terminal electron acceptor in respiration. Bacteria that are oxidase-negative (such as Enterobacteriaceae) may be anaerobic, aerobic, or facultative. The oxidase negative result just means that these organisms do not have the cytochrome c oxidase that oxidizes the test reagent. They may respire using other oxidases in electron transport. When present, the cytochrome c oxidase oxidizes the reagent tetramethyl-p-phenylenediamine to a purple color, and when there is no enzyme, the reagents remain reduced, and colorless.
History of bacteria in human urine.
For more than 60 years, scientists have thought that urine is sterile and that patients who test positive for bacteria in their urine have urinary tract disorders. By May 2014, a team of scientists from Loyola University announced that that might not be true, from studies done in 2012. Now, the team has used advanced methods to analyze urine specimens collected directly from the bladders of healthy women, and they found bacteria in urine. By using a technique called expanded quantitative urine culture (EQUC) and sequencing the subjects’ bacterial DNA, the team was able to identify bacteria not usually picked up by traditional urine cultures. This study also used 16S rDNA sequencing to classify bacterial DNA.
Brubaker's team analyzed urine samples from 90 women. It found bacteria both in the urine of healthy women as well as the urine of women with overactive bladder. However, the germs were different between the 2 groups. The study was presented in Boston at the annual meeting of the American Society for Microbiology. Findings presented at medical meetings are typically considered preliminary until published in a peer-reviewed journal.
Another team by June 2017 led by Wolfe that sampled 75 patients with UTI, using the old technique, and the new EQUC technique, showed about 110 bacterial species using the new EQUC technique, compared to about 55 bacterial species in the old technique.
The belief that urine was sterile has its roots in the 1950s, when epidemiologist Edward Kass was looking for a way to screen patients for urinary tract infections before surgery. Kass developed the midstream urine test (still used when you pee in a cup) and set a numerical cutoff for the number of bacteria in normal urine: not more than 100,000 colony-forming units (cell clusters on a culture dish) per mL of urine. A person tests “negative” for bacteria in their urine as long as the number of bacteria that grow in a lab dish containing the urine falls below this threshold.
As of Dec. 2019, the bacteria that live in the bladder that are normally in the urine don't grow at room temperature in air during a period of time, especially not in urine, as urine's not a very good growth medium. So the organisms that were discovered that live in the bladder, like to be in 5% carbon dioxide and 37 degrees C, in which many of them are strict anaerobes. In women, the largest group of individuals are dominated by Lactobacillus, followed by Gardnerella and Streptococcus, and a little bit of Staphylococcus. Men don't have a whole lot of Lactobacillus or Gardnerella.
Cyanobacteria that can do photosynthesis of IR light.
In 2013, professor Don Bryant of Pennsylvania State University discovered that some types of cyanobacteria can do photoacclimation past 700 nm, up to 800 nm, but his team did not publish until Jan. 2022. It was thought for many decades that 700 nm was the light limit for photosystem I and II.
(Are photosystem I and II in cyanobacteria the same as the photosystem I and II in plants? For the most part, yes. In plants, PSI and PSII are located in the thylakoid membranes of the chloroplasts. In cyanobacteria, they are located in specialized internal membrane structures called thylakoids, which are part of the cyanobacterial cell.).
In photosystem I, chlorophyll f absorbs far-red light, with about 85 chlorophyll a, where 7 of them turn into chlorophyll f. In photosystem II, of the 30 chlorophyll a, about 4 change into chlorophyll f and 1 into chlorophyll d. However, the exact number is a challenge for spectroscopists.
Allophycocyanin is a type of phycobiliprotein found in the phycobilisomes of cyanobacteria tht absorbs the red light and transfers the energy to chlorophyll a in the reaction centers of photosystem I and II.
Virology: non-structural proteins.
In virology, nsp stands for nonstructural protein, which is a protein encoded by a virus but not part of the viral particle.
There are 2 types of nsps, in capital NSP1 to NSP5/6 are for rotaviruses, and lower-case nsp1 to nsp16 are for coronaviruses. Genes encoding nsps are nsp1 to nsp10, and nsp12 to nsp16.
Similarly, for flaviruses (such as Dengue, Zika, West Nile, and Yellow fever virus) use NS1, NS2A and NS2B, NS3, NS4A and NS4B, and NS5.
For picornaviruses (such as poliovirus, enterovirus, rhinovirus, and Hepatitis A virus) use 2A, 2B, 2C, 3A, 3B, 3C, and 3D.
For alphaviruses (such as chikungunya, Sindbis virus, and Eastern equine encephalitis virus) use nsP1, to nsP4.
For astroviruses, use nsp1a and nsp1ab.
Arteriviruses (such as PRRSV and Equine arteritis virus) however, use the same system as coronaviruses, but nsp1 through nsp12, and noroviruses, use the same system as flaviviruses.
For most positive-sense RNA viruses, which is all of the listed above except for rotavirus (which is double-stranded), nsps are produced after the viral genomic RNA is translated into polyproteins, which are then cleaved into individual nsps by viral proteases. But for negative-sense RNA viruses and double-stranded RNA viruses, carry their own RNA-dependent RNA polymerase to transcribe mRNA from the viral genome.
Negative-sense RNA viruses do not directly translate their genomic RNA because it is not in the correct orientation for ribosomal translation. Instead, they carry a viral RNA-dependent RNA polymerase (RdRp) inside the viral particle. This polymerase transcribes the negative-sense RNA genome into positive-sense mRNA, which is then translated into viral proteins, including nsps.
Examples of negative-sense single-stranded RNA viruses:
For the Influenza A/B viruses encode PB1, PB2, and PA as part of the polymerase complex.
For measles virus and Nipah virus, produces nsps such as C and V proteins.
For Ebola virus and Marburg virus, their nsps include components like he L protein (polymerase).
For Rabies virus, encoded an L protein and P protein as part of its replicase complex, which functions similarly to nsps in positive-sense viruses.
For Hantavirus and Rift Valley Fever virus, encodes nsps like NSs an NSm.
For Lassa virus, nsps include the L polymerase and Z matrix proteins, which help with RNA synthesis and regulation.
News.
2/19/2024.
New antibiotic overcomes antimicrobial mechanisms.
Researchers have created a novel molecule, which has an improved ability to bind to bacterial ribosomes, with a completely synthetic system.
Antimicrobial resistance is a global public health crisis, but now, researchers at Harvard University have created a new antibiotic which overcomes the antimicrobial mechanisms that have rendered many modern drugs ineffective.
The team was led by Dr. Andrew Myers, Amory Houghton Professor of Chemistry and Chemical Biology, who commented that that their synthetic compound, named cresomycin, kills many strains of drug-resistant bacteria, including Staphylococcus aureus and Pseudomonas aeruginosa. He added: “While we don’t yet know whether cresomycin and drugs like it are safe and effective in humans, our results show significantly improved inhibitory activity against a long list of pathogenic bacterial strains that kill more than a million people every year, compared with clinically approved antibiotics.”
Cresomycin.
The novel molecule shows an improved ability to bind to bacterial ribosomes, the particle whose main function serves as the site of mRNA translation and protein synthesis. Most antibiotics disrupt the ribosomal function, but some bacteria have evolved mechanisms that stop these drugs from working.
The cresomycin compound draws upon the chemical structures of lincosamides, a class of antibiotics that includes the commonly prescribed clindamycin. Clindamycin, like many antibiotics, is made by semisynthesis, in which complex products isolated from nature are modified directly for drug applications. However, cresomycin is fully synthetic and has chemical modifications that cannot be accessed through existing means.
Ribosomal binding.
Bacteria can develop resistance to ribosome-targeting antibiotic drugs by expressing genes that produce ribosomal RNA methyltransferases. These enzymes block the drug components that are designed to latch onto and disrupt the ribosome, which ultimately stops the drug’s activity.
Dr. Myers and his colleagues engineered their compound into a rigidified shape that closely resembles its binding target to overcome this issue, giving it a stronger grip on the ribosome. They call their drug “pre-organised” for ribosomal binding as it does not need to expend as much energy conforming to its target as existing drugs must do.
Component-based synthesis, a method pioneered by the Myers lab, involves building large molecular components of equal complexity and bringing them together at late stages. This modular and completely synthetic system enables them to create and test hundreds of target molecules, significantly quickening the drug discovery process.
Part 2: fungal microbiology.
As of Feb. 2025, there are no anti-fungal vaccine that are clinically available. Part of that is due to that pharmaceutical companies are not very interested in anti-fungals, from an economic standpoint.
The world's largest fungal herbarium is in The Netherlands: the Westerdijk Fungal Biodiversity Institute, located in Utrecht. As of Nov. 2019, houses about 24,000 herbarium species. However, that is the largest for specializing exclusively for fungi, otherwise, the Royal Botanic Gardens, in Kew, UK, houses a larger fungarium.
Some diseases caused by fungi are rice blast, banana wilt, and white-nose syndrome in bats. About 70 to 80% of plant diseases are causd by fungi. Rice blast are caused by Magnaporthe oryzae, and banana wilt (Panama disease) are caused by the soil-born Fusarium oxysporum. In bats, white-nose syndrome is caused by Pseudogymnoascus destructans, and was 1st identified in North America in 2006, has infected up to 90% of bats since, killing millions in earthern North America. This decline has led to higher insect populations, and therefore higher pesticide use by farmers, which leads to adverse human health outcomes.
For fungal disease that affect humans, include cryptococcosis, which affects the brain.