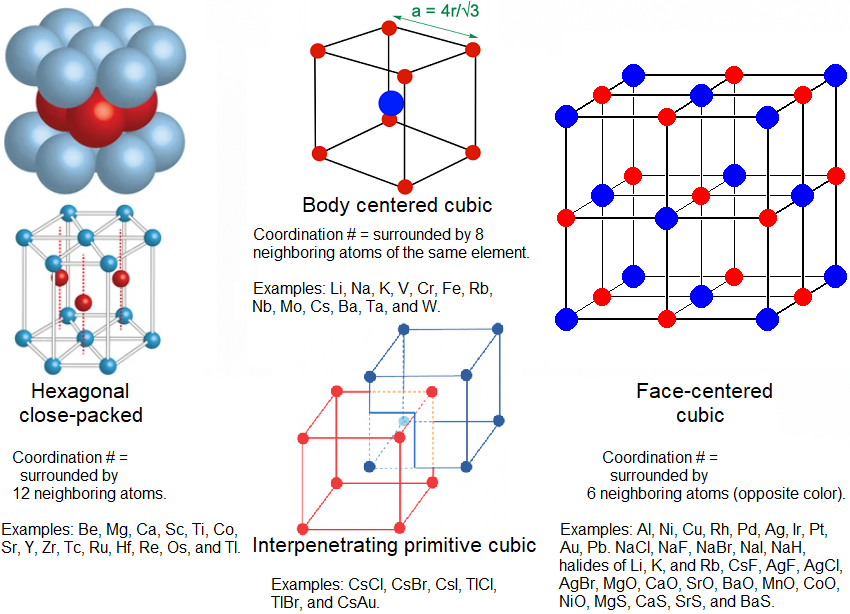
Lattice Geometry

This is a branch of inorganic chemistry. Can any metal bondings do 2 of the 4 geometries? Between the 1st 2 examples, some metals can switch between the 2 at different temperatures or and pressures. But at same temperature and pressure, they are at only 1 lattice structure.
The 3rd column is for ionic compounds, known as cubic close packing (ccp or fcc). Hexagonal close packing (hcp) and body centered cubic refer to the packing of atoms of the same size (typically metallic atoms), not alternating ions of different sizes and charges.
Elements that can do more than 1 lattice (polymorphs):
Ti at hcp lattice, undergoes a reversible change to bcc lattice at 882 C.
Sc from hcp to bcc at 1337 C.
Mn from hcp to bcc at 1143 C.
Fe from bcc to fcc at 912 C (at same pressure of 1 bar). But if you increase the pressure to 10 bar, is bcc as low as 527 C. Fe can also be hcp at room temperature at 11 GPa.
Co is hcp lattice, undergoes a reversible change to fcc lattice at 227 C, but back to hcp at 600 C.
NH4Cl and NH4Br from ipc to fcc at 184 C and 138 C.
The metallic radius also decreases with decreasing coordination #. The metallic radius is half of the distance between the nearest-neighbor atom in a solid state metallic lattice. So from a coordination # of 12, a coordination # of 8 has a metallic radius of 97%, 96% for coordination # 6, and 88% for coordination # 4. Pt and Au, have an estimated metallic radius of .139 and .144 nm, while Fe and Cu at .126 and .128 nm.
The 3 top lattice geometries in the image are obviously not the only kinds. Some geometries are so rare that only 1 known element (like Po) has it, and therefore the geometry is named after the substance. The 4th most common is known as the diamond configuration (coordination # of 4), which includes diamonds, Si, and Ge.
Difference between bcc and ipc:
Both have the same coordination # of 8. But bcc requires all the atoms to be the same element, whereas icp requires them to be 2 different substances bonded ionically: each cation species to be surrounded by 8 anion species, and each anion species surrounded by 8 cation species. Therefore, they do not have the same unit cell (the unit cells cannot be stacked). But for ipc, you can make 2 interlocking unit cells.
History: the 1st of each to be discovered.
HCP: around 1921, for Zn, Cd, Co-&betal, and Ru by Albert W. Hull.
BCC: 1916-17, for Fe by Albert W. Hull.
FCC: 1913, for Cu, Ag, and Au. NaCl and ZnS by William Henry Bragg and William Lawrence Bragg (father and son).
IPC: 1913, CsCl also by Bragg.
Diamond: also 1913 by Bragg.
In the same 1921 paper, Hull also identified Cr, Mb, and Ta crystallized in bcc form, and Ni, Rh, Pd, Ir, and Pt as fcc. Note: Max von Laue 1st demonstrated X‑ray diffraction in 1912, and won the Nobel Prize in physics for it in 1914.
Molecular shapes.
Aside from metal networks, there are individual molecules. Some of the common 4-coordinate (MX4) are tetrahedral and square planar.
Tetrahedral only: Zn(II), Mn(II), Co(II), Fe(III), Cd(II), Hg(II) Square planar only: Pd(II), Pt(II), Au(III), Rh(I), Ir(I) (all d8 metals) Both: Ni(II), Co(II) (sometimes), and Cu(II) (tetrahedral with weak-field ligands (Cl-) and square planar with strong field ligands (CN-))
The classic example of 8-coordinate molecules are called octahedral.
The Miller index.
In crystallography, {111} and {100} facets refer to specific crystallographic planes in a cubic crystal system. Crystallographic planes are described using Miller indices (h,k,l):
In Miller indices (h,k,l), the numbers (1, 0, 0), (1, 1, 1), etc. represent how a crystallographic plane intersects the unit cell axes in a cubic crystal system.
(100) plane: A flat surface perpendicular to one of the cubic axes.
(111) plane: A diagonal plane cutting through three axes at equal angles.
Miller indices (h, k, l) describe the orientation of a plane in a crystal. The values indicate:
1: The plane intersects that axis at 1 unit length.
0: The plane is parallel to that axis (does not intersect).
For a cubic crystal like gold (Au) or silver (Ag):
(100) Plane: Intersects only the x-axis (but not y or z)
(111) Plane: Intersects x, y, and z at equal distances, forming a diagonal cut.
{000} is not a valid Miller index because it would imply that the plane is infinitely far away (or doesn't exist at all). If all 3 indices are zero (0,0,0) means the plane never intersects any axis, this would represent no plane at all.
Miller indices are a general system used in crystallography for describing planes in any crystalline material, including:
-Bulk crystals (metals, semiconductors, ceramics).
-Thin films (used in microelectronics).
-Nanoparticles (gold, silver, etc.).
-Minerals (natural crystal structures).
How do we know that NaI favors nanotriangles, whereas NaBr favors nanocubes?
Atoms tend to rearrange to form lower-energy surfaces, preventing some exotic shapes from forming.
Iodide binds strongly to {111} facets, stabilizing triangular or plate-like shapes.
Bromide binds preferentially to {100} facets, promoting cube-like structures.
Iodide is larger and adsorbs more strongly, suppressing growth along certain crystal directions, leading to 2D structures like triangles.
Bromide has moderate binding affinity, allowing for more symmetrical 3D growth, resulting in nanocubes.