Colligative Properties
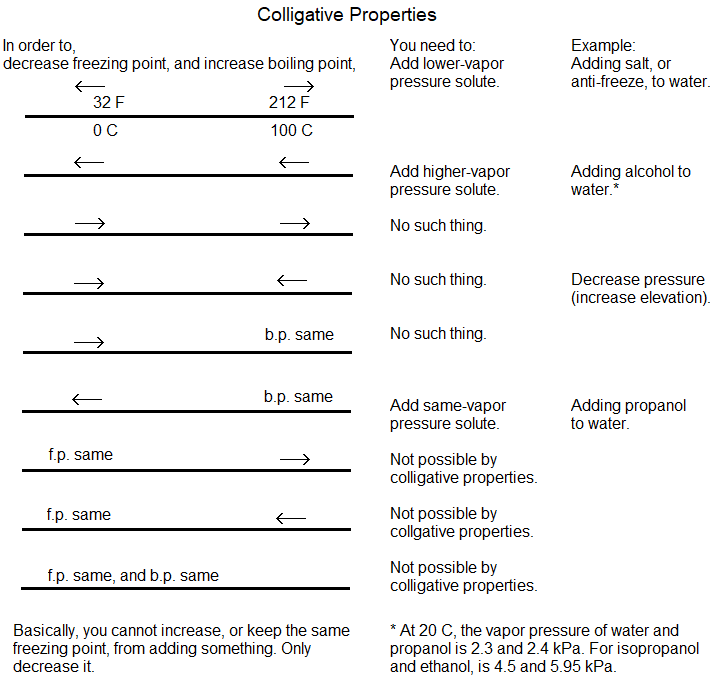
Can you keep a snowman in weather above the 32 F melting point? Not by colligative properties, and the below table will explain to you why.

Evaporation with temperature and humidity.
Water evaporates more with increasing temperature, but also evaporates less with increasing humidity. So is there any correlations where the increased temperature and increased humidity will make it evaporate the same?
Evaporation rate is proportional to the saturation vapor pressure (es(T), in kPa) at temperature T, multiplied by the dryness of the air (which is 1 - RH). RH stands for relative humidity. At 91 °F (33 °C), the saturation vapor pressure es(T) is approximately 5.0 kPa. At 20 °C, 2.34 kPa, and 30 °C, 4.24 kPa.
At 20 °C and 40% humidity, evaporation rate is similar to 30 °C and 67% humidity. At 91 °F and 59% humidity, water evaporates ~75% faster than at 68 °F and 50% humidity.
As you know, water evaporates more right after a rain, then right before. Evaporation is driven by the difference between water vapor pressure at the surface and the vapor pressure in the air. After rain, when air dries out, that difference is bigger, so faster evaporation. However, when the puddles evaporate, humidity is increased, slowing evaporation.
-
1. Say I have 1 gallon of water, how much does it evaporate in 1 hour, at 25 C and 30 C? Since the answer depends on the surface area, let's say the gallon of water is shaped into a cube.
1 gallon of water is 8.33 lbs or 3,778.42 g. A cube of a gallon of water is V = .003785411784 m3. A cube side, s = V1/3 = .15585 m, surface area A = s2 = .0242889 m2. [Area = s * s * s for length, width, ands height, so s3, therefore, s = V1/3].
Evaporation model, J = kes(T)(1 - RH), where J is the mass flux (kg/m2s2 * k/Pa).
Since the relative humidity wasn't specified, we'll use 50% (.50) which represents indoor value.
Since the vapor pressure wasn't specified, for 25 C and 30 C, we'll use the saturation vapor pressure, es, as 3.17 kPa and 4.24 kPa.
Since air flow (k) wasn't specified, we'll use very still air, and moderate airflow, k = 1.0 * 10-7 kg/m2s2 * k/Pa, and k = 5.0 * 10-7 kg/m2s2 * k/Pa.
Mass evaporate per hour = J * A * 3600 (kg/hour) and convert to grams.
At very still air, and 25 C, evaporates .01386 g / hour.
At very still air, and 30 C, evaporates .01854 g / hour, a 33.75% increase.
At moderate airflow, and at 25 C, evaporates .06930 g / hour.
At moderate airflow, and at 30 C, evaporates .09269 g / hour.
So the answer comes out to not just less than 1 g/hour, but less than .1 g/hour.
-
Note that evaporation itself is not a colligative property, but some things that affect evaporation are related to colligative properties.
Colligative properties depend only on the number of solute particles, not their identity. The 4 classical ones are:
-Vapor pressure lowering
-Boiling point elevation
-Freezing point depression
-Osmotic pressure
All 4 occur because adding solute particles reduces the escaping tendency of solvent molecules. So, evaporation is a kinetic property, while vapor pressure (and vapor pressure lowering) is a colligative property. Adding salt to water will slower the evaporation.