Chemistry: Research Trivia
%i. When you turn cold water from the faucet, the water looks clear. But when you turn hot water from the faucet, the water is foggy. What is that fog?
Calcium carbonate (CaCO3, limestone), which is formed from calcium ions and bicarbonate ions when heated. However, cooling back the water will not decompose it. (This is not the only way to make calcium carbonate.). Heating water also allows carbon dioxide to escape. This is mainly why it safer to drink and cook cold tap water, than cook hot tap water.
%i. When the bathroom sink is clogged, what’s a good chemical to pour down the drain?
Something with a strong base, particularly NaOH (lye). The acid versions in stores tend to be concentrated sulfuric acid. If you want to use something milder, you can buy HCl as toilet bowl cleaners in 8%, 10%, and even 24% HCl, or even as muriatic acid for pool pH adjusters and concrete cleaners, at 31.45% (20 baume).
%i. Why is it bad to mix bleach (NaOCl) and ammonia (NH3) or cleaning solutions of them?
NH3 and NaOCl form chloramine (NH2Cl), which is unstable and explosive.
Similarly, mixing bleach with isopropanol produces acetone, mixing bleach with ethanol produces acetaldehyde, both along with salt and water.
%i. What temperatures can roadsalt be used at?
NaCl works to temperatures as low as 21.2 F (-6 C), and CaCl2 as low as -25.6 F (-32 C). So most roadsalt is a mix of CaCl2 and NaCl.
%i. Why are potholes formed on the road during the winter?
The freeze-thaw cycle of water. Water goes into the road in the winter, freezes into ice, and therefore expands, causing pressure under the road. (Ice is therefore less dense that water.). (This also explains why water pipes crack at below freezing temperatures.). (The key compound in Portland cement is CaO, at 67%, then SiO2, at 22%, then Al2O3 at 5%, and Fe2O3 at 3%.).
The freeze-thaw cycle of water, for example, can also explain why berries must be kept above water's freezing point. So 33-35 F. If taken to below 32 F, then the water becoming ice, expands, causing physical damage to the insides of the berries (though this is in no way microbiological damage).
%i. What’s a good chemical to remove oil from keyboards?
Some good answers to remove oils in general are cleaning solutions containing alkane chains and ammonia, but those will react with the plastic keyboards. Something that won’t react with the keyboards are isopropyl alcohol, over ethyl alcohol, which can be bought at 70% and 91% concentration. While isopropyl alcohol is preferred over ethyl alcohol, ethyl alcohol solutions often contain acetone as a minor amount, which is a problem as acetone reacts with plastics. Another possibility is the gas can sprays containing difluoroethane, where the gas absorbs the oil from the keyboards, but the issue remains that you now have oily gas floating around. However, the oil from the fingertips is a fatty acid, so a good chemical to remove fatty acids is dichloromethane.
%i. What's a good chemical to remove paint, on unwanted surfaces?
For water-based paint, there's isopropyl alcohol and acetone.
For oil-based paint, there's mineral spirits, but those are poisonous to the nervous system.
If the can has words acrylic or latex, those are water-based. Of course you can buy later paint clean-up at hardware stores, their ingredients include acetone and d-limonene. If the paint is a paint and primer in 1, that can still be water-based or oil-based. The stain-blocking paint-and-primer in 1, made by Behr Ultra, are water-based.
%i. I turned my refrigerator off, or, the power went down, for a couple of days until the inside is room temperature. What’s the smear all over the inside walls?
Rancid fats.
%i. Can the same pans used on gas stoves be used on electric stoves?
No. Most pans, including stainless steel and cast iron, are compatible with induction, but some aren’t, such as those made of copper, aluminum, and anodized aluminum. copper and aluminum pans tend to heat up more quickly on gas stoves than on electric stoves. Materials like stainless steel and cast iron contain magnetic properties and work well with induction cooking. However, materials like aluminum and copper are not magnetic and won't work efficiently on induction cooktops unless they have a magnetic base or are layered with magnetic materials. Anodized aluminum, which is aluminum treated with an electrolytic process to harden the surface, typically doesn't work on induction cooktops unless it has a magnetic base.
Are there dangerous gases affiliated with gas stove? Yes, cooking with gas stoves creates nitrogen dioxide, which is a byproduct of fuel combustion and a known lung irritant, according to the U.S. EPA. NO2 exposures in homes have been associated with more severe asthma and increased use of rescue inhalers in children in previous studies. Use ventilation when cooking.
%i. For gas stoves, do you recommend Teflon, iron, or aluminum pans?
Teflon is the tradename or nonstock coating made from PTFE. Teflon pans used to have PFOA, which were phased out in the U.S. around 2013. If the coating has scratches or starts peeling, that is unsafe as small flakes of PTFE can mix with food. Teflon pans are generally safe when below 450 F (232 C), and can start to break down above 500 F (260 C), where the fumes are fatal to birds.
Alternatives are iron or aluminum pans. Iron pans are much heavier, slower to heat, but slower to cool down, whereas aluminum pans are lighter, quicker to heat, but quicker to cool down. Iron pans are better for long-term low-flame cooking. Aluminum better for quick cooking.
If you heat a Teflon pan at high flame for 6 minutes, will it hit 500 F? Yes, it can hit 500 F in 2-3 minutes when the pan has no oil or water. But with oil, food, or water, will slower it.
%i. Carbon monoxide gas detectors: how do we know CO absorbs IR and not N2?
Because CO is polar. The energy causes a change in dipole moment (the bond distance changes, because it is polar), but does not change the distance in N2. Where there is no change in the bond distance of a molecule, there is either no absorption of IR, or has a weak absorption of IR.
However, a molecule does not have to be polar in order to absorb IR. CO2 is non-polar and absorbs IR. The molecule has to have vibrational modes that alter the polarity. When vibrations change the dipole of a molecule, it is able to interact with electromagnetic radiation of the appropriate frequency. This is called spectroscopic selection rule. Oxygen and nitrogen do not absorb as they have no dipole, and they do not emit as they don't have dipole transition moments. All of them absorb UV light but not visible light.
%i. What’s the acid in our stomach?
It varies between .1 molar HCl (hydrochloric acid), at pH 1, to .03 M (pH 1.52). So swallowing nails, or anything with iron, dissolves.
But it is not safe to drink 10% or 8% HCl, which are the ingredients in toilet drainers. 10% HCl depends on whether you mean volume/volume or weight/weight. Sigma-Aldrich states their concentrated HCl to be 12.1 M, so 10% is 1.21 M, or pH of -.08, which is far more acidic than in our stomach. If you do weight/weight, so 100 g HCl / 1000 mL, so 100 g / 36.46 g/mol, = 2.74 M, or pH of -.437, that is even more acidic. 8% HCl comes to be .968 M or 2.19 M, or pH of .01 or -.34. Remember, pH scale is 10x, so a pH of 0 is 10x more acidic than pH of 1.
%i. Who else is less dense as a solid than it is as a liquid, besides water?
For elements, Ga, Ge, Sb, and Bi. Plutonium is like this too, however, after it melts, liquid plutonium expands upon heating. For compounds, hydrogen peroxide and paraffin.
%i. What’s a good way to detect lead in solid substances like pipes, without doing instrumentation?
You can buy a lead-detecting kit. You break it off, rub on the solid for up to 30 seconds (it’s a yellow color), and, if it turns red or pink, it has lead in it.
%i. How do we know why some metals are better conductors than other metals?
Copper wire is the 2nd best conductor element in the periodic table, 2nd to silver wire. Gold is 3rd. 1 component of resistance that is very important around room temperature is the scattering of electrons by collisions with the metal atoms in the lattice. The energy and frequency of these collisions are very hard to predict.
The answer has more to do with solid state physics than chemistry. As atoms are brought closer and closer together, their electron clouds overlap and interact. When a crystalline solid is formed, 1 abandons consideration of the atomic energy levels. Instead, 1 seeks out the Bloch functions to describe the electronic states. The Bloch functions explicitly consider the symmetries of the crystal. Further, they represent extended electron states, those that extend across the entire crystal. They represent the allowed (energy and momentum) combinations of electrons.
For a metal, the highest Bloch states occur as a band of states which is only partially filled. Unoccupied states lie just above states with electrons. To move an electron about, it needs just a little bit of energy, and off it can go. (Fully filled bands do not allow conduction - for any electron going 1 way there is 1 going the other way.).
In general then the conductivity should just be the number of electrons near the line between occupied and un-occupied. This line is called the Fermi surface, and things get more complicated quickly. The shape and connectivity of the Fermi surface impact conduction dramatically.
Copper is a simple noble metal with a beautiful near-ideal Fermi surface. It turns out that the Fermi surface of copper is almost exactly what 1 would expect from the free electron sphere: electrons can move easily in any direction, and the surface is fully connected.
Iron is much more complicated. Energy bands in ferromagnetic iron have 2 major differences. 1st, because iron atoms indeed have individual magnetic moments, the overall band structure is split into 2 different spin states for the electrons. So, each spin state is separated from the others, reducing how each can move (relative to copper, were such splitting does not exist). 2nd, and more importantly, the Fermi surface looks nothing like a free electron sphere. Instead, for iron the Fermi surface is not fully connected, comprising instead of multiple 'pockets' of electrons that do not communicate directly with the other pockets (and each spin state has a different set of pockets). To move electrons around, they have to 'jump' from one pocket to another through scattering. This makes it much harder to move the 'free' electrons in Fe around to get net conductivity.
%i. What are examples of inorganic drugs?
Examples of inorganic drugs, other than the antacids, are calomel (Hg2Cl2) and cisplatin (PtCl2(NH3)2). Cisplatin has been known as an anti-tumor drug since the 1960s.
%i. Is there such a thing as an inorganic oil, besides hydrazine? Silicone.
%i. What makes up a match?
The reactive chemicals on the tip of a “strike anywhere” match are usually P4S3 and an oxidizing agent such as KClO3. When the match is struck on a rough surface, the heat generated by the friction ignites the P4S3, and the oxidizing agent brings about rapid combustion.
Similarly, a gun is made of charcoal (S) and an oxidizing agent (KNO3).
%i. What kind of metals do magnets attract?
Permanent magnets are made of BaFe12O19. The most common elemental magnets are Fe, Co, and Ni. Gd is a 4th but much weaker magnet. The most common metal oxides that are ferromagnetic are CrO2 and Fe3O4. Some of the stronger magnets are molybdenum and neodymium.
In a ferromagnetic material, large domains of magnetic dipoles are aligned in the same direction.
Magnets do not work on coins or keys, as U.S. coins are made with copper and zinc, while keys are made with brass (brass is 2:1 copper and zinc). (A quarter and dime >1965 is 91.67% Cu, 8.33 % Ni, a nickel is 75% Cu, 25% Ni, and a penny >1982 is 97.5% Zn, 2.5% Cu.). So can you lift keys that fell down a tunnel, with a magnet attached to a rope? The keys won't, but the key chain can if it's the right material.
A Faraday cage is a shield against electrostatics, somewhat against RF fields. It has almost no effect against a magnetic field. You would need a shield of mu-metal to help against magnetism, but it would need to be very thick. Mu-metal is a nickel-iron ferromagnet, which is used for shielding sensitive electronic equipment against static or low-frequency magnetic fields.
The 1st time that ferromagnetism has been demonstrated in a gas, was in 2009, when physicists at MIT discovered lithium gas cooled to less than 1 Kelvin.
Any electron with a non-0 angular momentum will act like a tiny magnet.
%i. How do you make glass into mirror, and how do you make a mirror into glass?
The process is called silvering, and desilvering. Amateur astronomers have been silvering their own telescopes for centuries. The silver is deposited on the front surface of the glass (not the rear), where it tarnishes quickly and has to be redone. But in modern days, their mirrors are sent to professional companies who use vacuum deposits of aluminum, which tarnishes much more slowly.
%i. What are skunk scents?
Skunks spray thiols to protect themselves. Thiols are sulfur analogs to alcohols (having a SH group instead of an OH group). Skunk scent is composed mainly of 3-methyl-1-butanethiol and 2-butene-1-thiol, with small amounts of other thiols.
%i. What is natural gas made of?
Depending on the location, natural gas is about 70% methane, 10% ethane, 15% propane, and 5% butane. Natural gas is otherwise odorless, which is why tert-butylthiol (tert-butyl mercaptan (TBM)) is scented to it so it becomes smellable.
%i. What is gasoline made of?
Automotive gasoline is a complex mixture of hundreds of hydrocarbon predominantly in the C4 to C12 range. It also contains 5-7% toluene by weight, and 1-2% benzene by weight. There is no exact definition, also it depends on the season. Winter months have a higher amount of butane, due to being able to start a cold engine (therefore having a decreased density, making it less energy per gallon).
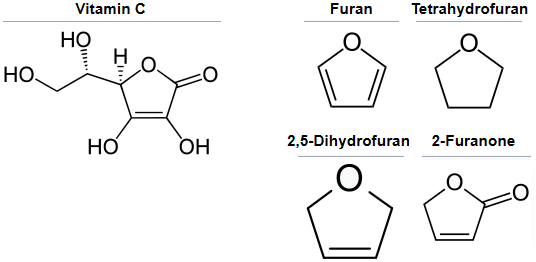
%i. What is the chief ring of vitamin C? Apparently it's not furan or tetrahydrofuran.
You are right about that, see the below image for the answer.
%i. What is the smell of sweaty coins?
When coins are touched with humans, the oils on our skin decomposes to 1-octen-3-one (octenone), which is responsible for the smell. Besides coins, includes irons metal, and blood. Similar to 1-octen-3-one, is 1-octen-3-ol, or known as octanol, which is contained in human breath and sweat.’
%i. What are the chemicals in fruits?
The compound responsible for the smell of garlic is allicin, C6H10OS2. The compound that gives the hot taste to chili peppers, is capsaicin, C18H27NO3. The foul smelling substance produced by the action of bacteria on meat is cadaverine, (CH2)5(NH2)2. The odor of pineapple is ethyl butyrate, and for bananas, isopentyl acetate, CH3CO2C5H11. For vanilla, vanillin. The principle compound in chocolate is theobromine (formerly called xantheose), C7H8N4O2.
Many monoterpenes are used in flavoring. For example, sabinene contributes to the spicy taste of black peppers, 3-carene to the smell of cannabis, and citral to the smell of citrus fruits such as lemon.
%i. How to make a dehumidifier? Using only chemicals?
Buy a bucket of calcium chloride, which can be bought at hardware stores. It can be used in moldy or wet basements. The chemical absorbs the moisture, so when the bucket is full of water, you empty it out. Calcium chloride was previously mentioned here as a powerful roadsalt.
%i. Can there be a compound that is polar and hydrophobic?
Hexamethylenetetramine. You can also have compounds with a polar hydroxyl group on 1 end, and a long hydrophobic tail on another end, such as the tocopherols.
%i. Can there be a compound that is non-polar and hydrophilic?
Yes, compounds like glycerol, tetrahydrofuran, and dimethyl sulfoxide.
Glycerol is a 3-carbon alcohol with 3 hydroxyl groups that make it hydrophilic, but the molecule itself is non-polar due to its long hydrocarbon chain. THF is a non-polar cyclic ether, but it is miscible in water due to the oxygen atom in the ring that can form hydrogen bonds with water molecules. DMSO has a polar sulfoxide substituent.
%i. Do we have something in chemistry where, A is generally more reactive than B, so can B react with something better than A does? To make this question more useful, let’s make A and B the same type of chemical, such as metals, acids, bases, etc.
The answer is yes. 1 principle example is if A is more reactive than B, but B is much smaller than A, then of course B can react faster if the reaction partner becomes more sterically hindered. For some specific examples with metals:
-Silver is considered more reactive than gold and platinum, as it tarnishes in air over time. But gold and platinum will dissolve in aqua regia (which is a 1:3 mixture of nitric acid and hydrochloric acid).
-Copper reacts with water and air much faster to make a green covering than aluminum. But aluminum will dissolve in acids more than copper.
%i. Can something be both radioactive, and photoluminescent?
Yes, but not typically with alpha radiation. (There is also no alpha radiation in the world of organic chemistry.). So with beta radiation, a radioactive version of any chemical that is chemiluminescent can be made simply by synthesizing it with a radioactive isotope of its atoms. All natural organic compounds that contain carbon-14 (which is the basis of carbon dating) are radioactive. Therefore, all fluorescence and phosphorescence compounds are radioactive, but at low levels.
Every element has radioactive isotopes, so you can always take a fluorescent molecule and replace some of it atoms with radioactive isotopes, to get both radioactive and fluorescent molecules (if the radioactive isotopes live long enough).
Examples.
Uranium glass is both fluorescent and weakly radioactive, and it lights up under UV light.
Paints made from a mixture of radium and a phosphorescing compound were once used in self-illuminating paints, at least until it was realized how toxic the radiation from the paints were.
Some luminous paints contain a source of radiant energy, such as salts of radium. These glowing paints are used for painting dials and signs to be read at night.
%i. What is the mechanism behind glow-in-the-dark toys?
The answer is not phosphorescence, but persistent luminescence. The chemical that emits the light is called the phosphor.
You can make your own luminescent paint with activated calcium sulfide, zinc sulfide, or other comparatively safe chemicals. Luminous paint (paint that glows in the dark because it contain a phosphor) will emit light for a certain time after exposure to an energy source, such as UV. (Calcium sulfide and zinc sulfide are both examples of phosphors, then, dope it to silver for blue, manganese for red, or copper for green.).
Persistent luminescence emission was 1st observed in the 17th century. In 1602, an Italian shoemaker, V. Casciarolo, observed strong luminescence from a mineral barite (BaSO4), later to be known as the famous Bologna stone.
%i. Can there be we mix 2 liquids and they emit light? Yes, called chemiluminescence.
%i. What chemicals kill bacteria but not viruses?
Triethylene glycol kills a lot of bacteria, but few viruses.
Ozone is more destructive to viruses than chlorine, but ozone is less soluble in water than chlorine.
%i. Is it safer to pee in a tub of water, or pee in a tub that is chlorinated? (As chlorine is anti-bacterial.).
It is worse to pee in chlorinated water, as the bacteria is not the issue. It is worse because now the urea and uric acid both react with chlorine, creating chloramines (from urea) and cyanogen chloride (from uric acid).
%i. If IR is spectroscopy for covalent bonds, then what is spectroscopy for ionic bonds? Use x-ray.
%i. In gamma decay, the radiation emitted has such a short wavelength it emits gamma radiation. Can there be a decay so weak in energy that it emits wavelengths longer than gamma rays?
Yes, thorium-229m, where m stands for metastable. In 1990, it was discovered to be less than 10 eV, so it can emit radiation in the x-ray and part of the UV. An eV of 7.8 corresponds to UV photons. It has a half-life of 7 microseconds.
%i. What is the glass of a smartphone?
It is an aluminosilicate glass that is toughened by soaking it in a bath of molten potassium nitrate (400 C), which compresses the atoms.
%i. How do catalytic converters work?
Platinum and palladium catalyze the oxidation of hydrocarbons and carbon monoxide, while rhodium acts as a catalyst for the reduction of nitrogen oxides. Palladium is the most common precious metal in exhaust catalysts, for the 2000s.
A catalytic converter consists of a honeycomb ceramic structure coated in Al2O3 (the washcoat), and typically operate at >90% efficiency. Catalytic converters require a temperature of 400 C (752 F) to operate efficiently. At its "light off" temperature, typically 347 C (656 F), it operates at only 50% efficiency, but as of 2008, the lead time of 90-120 seconds, the exhaust emissions are not controlled.
Note that because hybrid cars do not heat as much as gasoline cars (as engines of hybrid cars only run part of the time), hybrid cars catalytic converters require more precious metals. This made catalytic converters of the Toyota Prius hybrid a big target for theft.
Platinum has been widely used in the production of catalytic converters since they were mandated for all cars in 1975 in the U.S. and 1993 in the European Union and U.K. Gasoline engine catalytic convertor use about 5 grams of platinum.
%i. How can you identify metal?
For instrumentation, use XRF spectrometer. For reacting with dangerous chemicals, you react with an acid, then with a base.
Dissolve the metal pieces in dilute nitric acid (at least 6 M),
if it turns blue, then copper is present (Cu(NO3)2.
then treat an aliquot of the resulting liquid with ammonia:
if it turns white, then it is lead or bismuth.
then treat an aliquot of the resulting liquid with sodium hydroxide (less than 2 M):
if it turns green, then iron(II) is present (Fe(OH)2).
if it turns white, then aluminum is present (Al(OH)3).
then if put in a iodide salt solution,
if it turns yellow, then lead is present (PbI2).
if it turns white, then silver is present (AgCl)
Dissolve in hydrochloric acid,
if it turns white, then silver is present.
Of course, with Cu, after Cu(NO3) is formed, you can continue with a base to get Cu(OH)2, but that is less blue of a color.
With iron, if you used more than 6 M nitric acid, you will get Fe(III) rather than Fe(II) (and therefore get Fe(OH)3.
With aluminum, if you used more than 6 M nitric acid, you will form Al2O3 to form on the outer surface, which will end the reaction.
%i. What's a chemical I can use to pour on dog crap to neutralize it?
This is going to be a microbiology question. Certainly chemicals like hydrogen peroxide and bleach will kill bacteria and viruses, but if the dog crap is on grass, the chemicals are not going to do the grass any good.
Matter cannot be created or destroyed, but 1 way is to speed up bacteria to eat the dog poop. Outside of a lab environment, increasing the oxygen concentration is out of the question. So for something specific to speed up bacteria eating the dog crap (and releasing it as gas) is coffee grounds. Sprinkling sugar onto the dog crap won't help much as sugar doesn't contain nitrogen. A category of something to sprinkle, are compost activators or and microbial inoculants, found in garden centers. Used coffee grounds can be added to a compost pile as a compost ingredient, and can improve the soil by feeding the soil microbes.
It is true that some rain will also speed up bacteria eating dog crap, due to the moisture, but if too much flooding decreases the oxygen content, then it will slow down the process. So on a non-rainy day, sprinkle some water and coffee grounds on dog crap.
Aditionally, adding coffee grounds are good for acid-loving plants (blueberries, azaleas, rhododendrons), slug and snail deterrent, carrot and radish growth (improving root developments), and attracting earthworms. Coffee grounds are bad for tomatoes and peppers (stunts growth), seedlings, lawns (promoting fungal growth), and herbs that don't like acidity (lavender, rosemary).
%i. Is it true when you buy new clothes from a store, you have to laundry it because of the chemicals on it that weren't washed?
Yes, especially for colored t-shirts and flannels, due to dyes/colorants and formaldehyde. Formaldehyde is used to prevent wrinkles and shrinkage, as well as mildew resistance, which prevents mold and mildew during storage and shipping. So exceptions to these are generally socks and pants that are black/white/gray.
Large textile industries generally do not wash clothes before they are shipped to stores, as washing every item before shipping would increase costs and time.
Environmental chemistry.
%i. Why is it thermal pollution when the temperature of water increases?
The solubility of gases decreases with increasing temperature. If a glass of cold tap water is warmed, bubbles of air are seen on the inside of the glass. For carbonated beverages, the solubility of CO2 decreases and therefore escapes from the solution. For the decreased solubility of O2 in lakes, warm water is less dense than cold water, causing warm water to float on top of cold water. This impedes the respiration of aquatic life at the bottom.
%i. What chemicals are found in tap water?
In Chicago, and according to the EWG (Environmental Working Group), some chemicals found in tap water are: chloroform (9.57 ppb), bromodichloromethane (7.37 ppb), dichloroacetic acid (5.19 ppb), trichloroacetic acid (4.45 ppb), dibromochloromethane (4.15 ppb), dibromoacetic acid (.652 ppb), and hexavalent chromium (.194 ppb). With the exception of the last 1, the rest are formed due to disinfection with chlorine gas.
A pitcher filter system, such as Brita, advertises as removing lead, cadmium, asbestos, and chlorine. The main ingredient is a coconut-based activated carbon, which reacts with chlorine, and an ion exchange resin, which removes lead and heavy metals. However, if you don't change your filter in time, bacteria can build up.
Some organic nutrients found in tap water include beta glucan.
%i. If I boil cold tap water, and cool it back to room temperature, is that safer than not having boiled it?
For bacteria in water, yes, but will not affect much chemicals in water. Only a few will boil into a gas, such as chloroform (61 C), bromodichloromethane (90 C), but will not affect dibromochloromethane (120 C), dichloroacetic acid (194 C), trichloroacetic acid (197 C), or dibromoacetic acid (decomposes 234 C).
%i. Is it safe to drink rain water?
Before we talk about the 2019 discovery of "forever chemicals" found in rain water, we will go back in order.
According to a 1962 publication from the U.S. Geological Survey by Dorothy Carroll, titled Rainwater as a Chemical Agent of Geologic Processes, A Review, rain water has sodium, potassium, magnesium, calcium, chloride, bicarbonate, and sulfate ions as major constituents, and minor constituents as iodine, bromine, boron, iron, alumina, and silica (Hutchinson, 1957).
According to a Nov. 2004 study from Carleton College of rain water collected in the U.S. from 48 sites in 31 states (including Alaska and Hawaii), they analyzed 7 anions using ion chromatography. Chloride, nitrate, and sulfate were found everywhere, with very few places had nitrite and phosphate, and no where had fluoride and bromide.
But the 2019 discovery of forever chemicals, changed that. Forever chemicals are a nickname for per- and polyfluorinated alkyl substances (PFAS). This new research found that rain and snow all over the world has them, and therefore rain anywhere is unsafe to drink. However, according to the map on the EWG website, forever chemicals are still not distributed evenly across the U.S. Some of the most heavily-contaminated places for forever chemicals found in drinking water are in North Carolina (where Chemours chemical plants are in), northern Alabama, New Jersey, eastern Massachusetts, and parts of California. www.ewg.org/interactive-maps/pfas_contamination/
Unfortunately, almost all of them boil above water, so boiling water does not help. This includes a basic 1, polytetrafluoroethylene (PTFE) at 327 C. For perfluoroalkyl carboxylic acids (PFCAs), only the simplest 1 with small molecular weight can be boiled, such as trifluoroacetic acid (CF3COOH, 72.4 C), but not perfluoropropanoic acid or perfluorobutanoic acid (C2F5COOH and C3F7COOH, at 96-97 C and 120 C), as well as the perfluorosulfonic acids (PFSAs).
%i. Can activated-carbon remove PFAS? (In a water filter.).
Granular-activated carbon has been shown to effectively remove PFAS from drinking water when it is used in a flow through filter mode after particulates have already been removed. It works well on longer-chain PFAS like PFOA and PFOS, but shorter chain PFAS like perfluorobutanesulfonic acid (PFBS) and perfluorobutyrate (PFBA) do not adsorb as well. Unfortunately, there are no obvious signs of filter exhaustion unless, without using LC-MS at home.
%i. Distillation: how efficient is boiling water? (such as desalination.).
Apparently not very efficient. According to an engineering video, a 500 mL flask of salt water, at about 300 mL filled, was heated on a sand bath on a hot plate, and used a Kill-A-Watt meter to track heat used, at home. Ice water is used as a condenser. But it took almost 2 hours to get 200 mL of water, which is about a kilowatt-hour of electricity. So 10 hours/liter at 5 kW-hour/liter. That is about $800/day for 300 gallons/day, which is an estimated U.S. household water usage. (However, distillations done at plants are much more efficient.).
A workaround plants use is a flash evaporator. Those send a liquid stream through an expansion valve to force water to evaporate at temperatures lower than their boiling point. This is the workhorses of desalination plants that use distillation. The other method desalinations plants use is membranes (reverse osmosis).
%i. Can I make a material that absorbs and traps carbon monoxide? And use it in my garage?
No. Such a material would be called metal-organic framework. As on 2023, some universities have experimented with Fe(II)-based metal-organic framework, where Fe(II) is the iron in hemoglobin. Whether or not these MOFs have been efficient or not, is another question. So it is not something you can buy in a store yet. It appears more universities have put more effort into using MOFs to absorb carbon dioxide instead. Cyclodextrin MOFs has been reported to (albeit reversibly) capture CO Food chemistry.
%i. How can you tell if a restaurant's hotdogs contain nitrates and nitrites in them, without asking?
You can assume they are in them. Nobody would put sodium nitrate and nitrite on hotdogs right before cooking them, they were put in as a preservative long before (as well as on deli meats). Hotdogs don't tend to be cooked like burgers, but taken out of a hot water bath. So it becomes a question of how often do they change the water bath.
Somewhat of good news is, sodium nitrate and sodium nitrite are both more soluble than salt is, in terms of rinsing hotdogs. Per 100 g, salt dissolves in water about 36 g at room temperature (25 C). Compared to sodium nitrate and sodium nitrite at 89 and 85 g. However, that can only rinse nitrites off the surface of hotdogs, they can still have nitrates and nitrites inside the hotdog during the curing process. So there will be nitrates and nitrites digested into the body, which leads to nothing but bad news. They both can raise blood pressure, increase colon cancer, as well as form into NOCs (N-nitroso compounds). In the intestines, bacteria reduce nitrates into nitrites. Nitrites are also formed from esters of nitrous or nitric acids in reaction with glutathione (but this reaction can also form methemoglobinemia). Nitrites are also formed from peroxynitrite (ONOO-) (it has a half-life of about 1 second, but it can be detoxified by glutathione peroxidase and peroxiredoxins, into nitrites (ONO-. In acidic urine, nitrite salts can be converted to nitrous acid, but nitrites can still be found in urine.
Organic hotdogs are also contaminated (with pesticides) if it contains celery juice that is not organic.
%i. What are the dangers of cooking potatoes?
Cooking starchy foods, such as potatoes, at high temperatures (120 C or higher) causes a chemical reaction between certain sugars and the amino acid asparagine (which is found in starchy foods). This reaction forms acrylamide. And therefore longer cooking times and higher temperatures increases the amount of acrylamides. Thus crunch, dark brown potato fries are likely to contain higher amounts of acrylamide than non-crunch, non-brown. Most acrylamide is formed in the final stages of baking, grilling, or frying as the moisture content of the food falls and the surface temperature rises.
As of Feb. 2018, dimethylpolysiloxane is added to McDonald's oil before they cook their fries.
Chemistry lecture questions.
%i. Magnesium sulfate vs. ammonium nitrate in water. 1 turns hot in water, and the other turns cold in water. Why is that?
Electron-deficient electrons in the valence theory, tend to readily undergo hydration during dissolution, which release energy. They can form bonds with water molecules, but not ones where the cations are electronically satiated, where they cannot accept any water molecules. Even if they can form hydrogen bonds with water, that does not release energy the same way hydration does. However, see the next question's answer for another answer.
%i. Why are most combination reactions exothermic, and some endothermic? (And therefore the opposite for decomposition reactions.). Similarly, why are most combination reactions have negative entropy, and some positive?
These 2 questions, as well as the previous question, are all asking the same question.
And the answer to these (3) questions, is Gibbs free energy: "It is impossible to predict S (entropy) and H (enthalpy) a priori, without any computer quantum calculations."
This also explains why a something dissolves better in hotter water, or colder water.
%i. What happens when you mix an acid with a base?
If the base is a hydroxide base (like NaOH) or an oxide base (like Na2O), then you get water and an inorganic salt. But not if the base is a hydride base (like NaH), then you don't get water, you get an inorganic salt and hydrogen gas. However, if the hydride base is with an acid that is also an oxidizing agent, then you get the inorganic salt, a gas, and water.
%i. Can there be an ionic salt, that is also a liquid, at room temperature?
For organics yes, those are called ionic liquids. But ionic liquids are not inorganic. Some of the ionic inorganic salts with the lowest melting points are lithium iodide (876 F), lithium nitrate (491 F), and lithium nitrite (432 F). That's 469 C, 255 C, and 222 C.
%i. Why can carbon form bonds with hydrogen, but not sodium?
Carbon does form 1 bond with Na, but poorly. Carbon forms covalent bonds with hydrogen because they are both non-metals. But metals do not like to form covalent bonds, so carbon poorly forms ionic bonds with sodium. Metals generally form ionic bonds.
In methane, the C-H bond is low difference in electronegativity. In methyllithium, the electronegativity difference is high, and so the bond is highly polarized towards carbon, because the 2s orbital of lithium isn't too high. It reacts violently with water and alcohol, For methylsodium, also high electronegativity difference. The 3s orbitals is already energetically too high to form bonds with a significant covalent part. This isn't soluble in most solvents, the same can be said for K, Rb, and Cs compounds.
%i. Why is iron sulfate a reducing agent, but iron nitrate an oxidizing agent? Copper(II) nitrate is oxidizing, while copper(II) sulfate is a weak oxidizing agent.
Due to the smaller charge on the cation. For iron, iron(II) is reducing, while iron(III) is oxidizing.
So while iron(II) sulfate is a reducing agent, there is a iron(III) sulfate, which is a weak oxidizing agent.
%i. How are we supposed to know that Cu(I) and Fe(II) favor tetrahedral geometry, and Cu(II) and Fe(III) favor square planar and square-pyramidal geometry?
If the question is in context of Cu(0) bonded to other Cu(0) metals, or Fe(0) bonded to other Fe(0) metals, then the question is false, and will be addressed at the bottom.
For coordination compounds:
The less and less electrons, the more space for forming a ligand. So the larger the cation charge, the larger the coordination number, to form bonds. So metal cations with higher oxidation state, form higher coordination geometry.
Lower oxidation states, such as Cu(I) and Fe(II), often exhibit a preference for tetrahedral coordination geometry (4-coordinate), especially when the d-orbitals are fully filled (d10 for Cu(I) and d6 for Fe(II)). This preference arises because there are no unpaired electrons to participate in bonding, leading to a geometry that maximizes the separation between ligands.
In tetrahedral coordination, this preference arises because there are no unpaired electrons to participate in bonding, leading to a geometry that maximizes the separation between ligands. When all the d orbitals are fully occupied, then there are no d-electrons to participate in d-orbital overlap with the ligands. As a result, the geometry tends to maximize the separation between ligands, which is achieved in a tetrahedral (4-coordinate) arrangement.
Higher oxidation states, such as Cu(II) and Fe(III), can exhibit a wider range of coordination geometries due to the presence of partially filled d-orbitals, which can participate in bonding with ligands.
An example of a tetrahedral Fe(II) complex is [Fe(CN)4]2- (tetracyanidoferrate(II)).
Square planar and square-pyramidal are not common for Fe(II). Those examples are more complex.
But neither of those are steel. In steel, iron atoms typically exist in a metallic bonding environment, where they are surrounded by a sea of delocalized electrons rather than specific ligands.
And neither of those Cu(0) are Cu(0) bonded to other Cu(0). Cu(0) metal bonded to other Cu(0) metals are face-centered cubic (FCC) crystal structure, where each Cu(0) ion is surrounded by 12 nearest neighbor Cu(0) ions, resulting in a coordination number of 12. Cu(I) can form with other Cu(I) in certain structures such as metal clusters and aggregates. Cu(II) does not form metals with itself.
%i. How are we supposed to know the oxidation state in methane are -4 for carbon and +1 for the 4 hydrogens, rather than +4 for carbon and -1 for the 4 hydrogens?
Don't confuse formal charge with oxidation state. The formal charge for everything in methane is 0. As for oxidation state, the reason carbon is given -4 and the hydrogens as +1 is due to carbon being more electronegative than hydrogen. Oxidation states are just a bookkeeping to track which atom the electrons are closer to.
Formal charges do not apply to ionic compounds. The formula for formula charge is (valence electrons) - (# of lone pairs) - (1/2)(bond electrons), but this formula doesn't apply to ionic compounds.
%i. Why are nitrogen and oxygen gas colorless, yet chlorine and fluorine gas are colored? Why is oxygen colorless, when liquid and solid oxygen are colored?
Color results from the electronic transitions between the HOMO and LUMO orbitals. For chlorine and fluorine gas, the absorption is in the visible range. The HOMO and LUMO gap is larger for oxygen and nitrogen.
Note that oxygen gas is weakly light blue. That has to do with requiring 2 oxygen molecules with 1 photon, which is hard to achieve outside a condensed phase.
Why is oxygen at the concentrations found in the atmosphere colorless, despite having absorption lines in the visible region?
(For O2, not O). The blue color of oxygen in the liquid and solid state are due to electronic transitions by which molecules in the triplet ground state excites to the singlet states. These transitions are normally forbidden in the gas phase, but can occur in the IR spectrum at 7918/cm and 13195/cm. However, in the condensed phase, a single photon can elevate 2 colliding molecules simultaneously to excited states, thereby requiring energy absorption in the visible region.
%i. Why does gold and platinum not react with concentrated nitric acid or hydrochloric acid, but if you mix the 2 together, to aqua regia, aqua regia now dissolves gold and platinum?
Because nitric acid and hydrochloric acid react with each other to form NOCl (nitrosyl chloride), which reacts with the gold and platinum. But describing it as NOCl + Au --> AuCl4 + (NO)4 in a single step is still a simplification of what's actually happening. There's a few other reactions going on with aqua regia, such as chlorine gas being formed. For platinum, reacts to form nitrosyl hexachloroplatinate(IV), 6NOCl + Pt --> (NO)2[PtCl6] + 4NO.
%i. Iron (the principle component of steel) melts at 1,538 C. If you heat iron to almost its melting point, does it get softer, or is it still like it is as room temperature?
Yes and no. There are phases where iron gets softer then harder, though softer is not the right word.
At room temperature, the iron atoms are in an unusual loosely packed open arrangement. As iron is heated past 912 C, the atoms become more closely packed before loosening again at 1,394 C.
Iron starts to glow dark red at around 800 C, or cherry red around 840 C (blackbody radiation). Blacksmiths are known to hammer iron during those colors, which would make a waste of effort if iron is not already softened at this temperature.
Iron is magnetic at room temperature, and previous work predicted that iron’s magnetism favors its open structure at low temperatures, but at 770 C iron loses its magnetism.
Solids store heat as small atomic vibrations, vibrations that create disorder, or entropy. At high temperatures, entropy dominates thermodynamics, and atomic vibrations are the largest source of entropy in iron.
%i. Are there positively charged ligands which can bind to a central metal atom to form coordination compounds? Ligands are Lewis bases which donate a pair of electrons, and the central metal atom is usually a Lewis acid.
Yes, aromatic cations like the tropylium (C7H7+) or cyclopropenyl (C3H3+) are well known aromatic carbocations that can coordinate to metals, creating sandwich or half-sandwich compounds. The positive charge is shared by all carbon atoms of the rings. The nitroso ligand (NO+) is also 1 of the most commonly occurring positively charged ligands. There's also H2NNH3+ (hydrazinium) and NO2+ (nitronium).
As far as ligand accepting electrons from central atom, such ligands do exist and are called Z-ligands, as opposed to "normal" ones classified as L-ligands, or X-ligands. They are Lewis acids, but usually still neutral. Another issue is pi backbonding, which also shows bonding from central atom to ligand.
%i. How far can electron-transfer reactions happen?
Typically can happen up to 14 Angstroms apart, but Lewis acid-base reactions will often require less.
In proteins and enzymes, electron transfer can occur over longer distances. This is due to the unique structural and functional features of proteins, including the presence of metal centers, cofactors, and mediating residues, which facilitate and stabilize long-distance electron transfer. Certain amino acid residues within the protein can act as intermediate electron carriers, effectively bridging the gap between the donor and acceptor sites. These residues can form a relay system that allows electrons to hop across longer distances. Electron transfer in proteins can also involve quantum tunneling, where electrons pass through potential energy barriers, and hopping, where electrons move stepwise via intermediate states.
For blue copper proteins, electron transfers can happen at 18 Angstroms apart for quantum tunneling and 34 Ansgstroms for combined quantum tunneling and hopping.
%i. What are examples of reactions that are thermodynamically favored, but not kinetically favored, or are kinetically favored, but not thermodynamically favored?
For the 1st, are reactions that have a negative Gibbs free energy (ΔG < 0) (spontaneous), but they proceed slowly due to a high activation energy barrier.
Examples include the conversion of diamond to graphite (graphite is more stable at standard conditions, but this conversion is extremely slow due to a very high activation energy barrier), rust formation (Fe + O2)2 -> Fe2O3, and conversion of ozone to oxygen.
For the 2nd, are reactions that are non-spontaneous (ΔG > 0), but have low activation energy so therefore the reactions happen quickly.
Examples include the conversion of graphite to diamond, but under high temperature and high pressure, liquid water below 0 C (water can remain liquid below its freezing point if there is a lack of nucleation sites for ice formation), and metastable allotropes of elements, such as white P resisting conversion to the more stable red P due to an activation energy barrier.
Inorganic chemistry.
%i. Crystal field theory and ligand theory are for d transitions. What about for f transitions?
Ligand field is established for f orbitals also, but it’s a much weaker perturbation so not given too much consideration. There are definitely f-related transitions that can happen, but they aren’t as influenced by geometry as they are for d orbitals since the bonding is essentially ionic in nature by the time you invoke f orbitals in a meaningful context.
Energy is very small for lanthanides so you don’t worry too much about ligand field effects. The radial distribution of the 4f orbitals is so small that it is treated as “core-like” - the interesting photophysics for lanthanides come from electron-electron repulsion and spin orbit coupling, ligand field effects only weakly modify that picture, unlike d-orbitals. Ligand field can be stronger for actinides because 5f has larger radial distribution comparing to 4f elements, but biggest effects are still the electron-electron repulsion and spin orbit coupling.
For some more details, if you look at radial extension of orbitals and compare 4f and 5f orbitals, you'll see that 4f orbitals are all closer to the nucleus than the xenon orbitals before it, while the 5f overlap and extend beyond the radon core. Any contribution from d-orbitals can greatly increase the transition intensity, f-f transitions are forbidden (as are d-d transitions without ligand field effects), but f-d transitions are not Laporte forbidden. Meanwhile, since ligand effects are minimal for pure 4f systems, there is very very little relaxation for these elements.
The early actinides (Pa-Pu) have significant d-f degeneracy, and thus experience much more intense coloration than congener lanthanides. Moreover, as 5f electrons are not fully shielded by the radon core of electrons (due to relativistic effects), they experience much greater ligand field effects than 4f elements, though still modest by comparison to many transition metals.
Photochemistry.
%i. Can something absorb UV and emit IR, or absorb IR and emit UV?
Absorbing UV and emitting IR is the spontaneous direction, for Stokes shifts. Absorbing IR and emitting UV is anti-Stokes shifts, or upconversion, which is uncommon.
So glow-in-the-dark toys emit regular light from sunlight in the UV and regular light wavelengths. However absorbing IR and releasing it into UV or regular light, is very improbable. In the field of quantum dots however, a new area of research is microwaving quantum dots, which will cause it to release light.
%i. When was the 1st time scientists discovered phosphorescence and anti-Stokes shifts?
That may be surprisingly recent. Doing a search for phosphorescence, and upconversion, in Web of Science, and SciFinder, the earliest papers that mention both started in 2000. Most of these papers refer to rare-earths, but sprinkled in the papers are ones that involve molecules.
Now, the challenge of finding a molecule that can do both phosphorescence and upconversion at the same time, is a bit of a challenge, and a contradiction, as upconversion requires hotter temperatures, while phosphorescence usually requires colder temperatures.
Regarding fluorescence and upconversion, vs. phosphorescence and upconversion, it should be noted that for molecular solar thermal management materials, scientists look for fluorescence molecules that can do upconversion, as phosphorescence and upconversion is inefficient. For the process where you use a sensitizer, then an annihilator, the sensitizer should be fluorescent-sensitive.
Medicinal chemistry.
%i. How can we tell that a chemical opens or closes ion channels?
From looking at the structure, you cant't. It is however, more easier to predict reactivity with a receptor than with ion channels. This study is called SARs (Structure-Activity Relationships) between compounds and their targets. As stated, ion channels are hard to predict, receptors more easier. It's about the ease and possibility of crystallizing the protein in question to be able to study it using X-ray crystallography and similar techniques. The function of an ion channel depends on the lipid membrane that you can't exactly crystallize, while most receptors don't. There have been some novel workarounds for that, but in general you tend to have much more data on the 3D structure of receptors than proteins whose function is defined by their introduction of a gradient on 2 sides of a biological membrane.
Pharmacology.
%i. I am overweight. What kind of drugs can I take?
There is no inorganic secret to obesity. Reportedly, there are overweight inorganic chemistry professors as well as billionaires that are overweight.
But there are drugs out there in the market relatively recent for that. Unfortunately, prescription drugs for obesity tend to be regulated to people who have a BMI of over 30, or over 27 with at least 2 comorbidities (such as hypertension and diabetes). You have appetite suppressants (anorexiants) such as phentermine and diethylpropion. But there is only 1 lipase inhibitor in the market, which is orlistat. There is both a prescription and non-prescription version of it. In the U.S., orlistat was 1st approved by the FDA in April 1999 for prescription-only (Xenical). On Jan. 23, 2006, an FDA advisory panel voted 11 to 3 to recommend approval of an over-the-counter version of orlistat (Alli by GlaxoSmithKline). Approval was granted on Feb. 7, 2007, and available in the market in June 2007, as 60 mg capsules, which is half the prescription dose.
Orlistat is a pentanoic acid ester that inhibits gastric and pancreatic lipases, thus decreasing the breakdown of dietary fat into smaller molecules that can be absorbed, decreasing fat absorption by about 30%. However, orlistat interferes with absorption of fat-soluble vitamins and beta-carotene, so patients are advised to take vitamin supplements of vitamin A, D, E, and K, but to be taken before or after 2 hours of taking orlistat.
Another set of medications are GLP-1 agonists are a class of medications that mainly help manage blood sugar (glucose) levels in people with Type 2 diabetes. Some GLP-1 agonists can also help treat obesity. GLP-1 agonists are most often injectable medications, meaning you inject a liquid medication with a needle and syringe. You give the shots in the fatty tissue just under your skin (subcutaneous injection). Areas of your body you can give the injections include your belly, outer thighs, upper buttocks, and the back of your arms.
The U.S. FDA approved the 1st GLP-1 agonist (Exenatide) in 2005. GLP-1 agonist medications currently available on the U.S. market include:
Dulaglutide (Trulicity).
There’s also a similar class of medications called dual GLP-1/GIP receptor agonists. As of spring 2024 there’s currently 1 of these medications on the market, called Tirzepatide (Mounjaro).
GLP-1 is a hormone that your small intestine makes. It has several roles, including triggering insulin release from your pancreas: blocking glucagon secretion, and slowing stomach emptying.
Obesity causes adipose tissue expansion and remodeling due to adipocyte hypertrophy and hyperplasia, which requires coordinated angiogenesis to provide nutrients and oxygen to all cells in the adipose tissue.
%i. What's a drug to counter motion sickness?
Besides ginger, along with the antimuscarinic agent scopolamine, certain antihistamines (H1-receptor blockers), such as diphenhydramine, dimenhydrinate (a chemical combination of diphenhydramine and a chlorinated theophylline derivative), cyclizine, meclizine, and promethazine, are the most effective agents for prevention of the symptoms of motion sickness. However, they are usually not effective if symptoms are already present and, therefore should be taken prior to expected travel. The antihistamines prevent or diminish nausea and vomiting mediated by both the chemoreceptor and vestibular pathways. The antiemetic action of these medications seems to be due to their blockade of central H1 and M1 muscarinic receptors. Meclizine is also useful for the treatment of vertigo associated with vestibular disorders. Combinations of antihistamines with decongestants are effective when congestion is a feature of rhinitis.
%i. What's a drug to help with sleep?
Melatonin is a hormone secreted by the pineal gland that helps to maintain the circadian rhythm underlying the normal sleep–wake cycle. Melatonin receptors are G protein-coupled receptors. Ramelteon (Rozerem) is the 1st melatonin receptor agonist to promote sleep, especially in sleep-phase disrupted sleep. Ramelteon was approved by the FDA in 2005, and was produced by Takeda Pharmaceutical Company. Ramelteon is indicated for the treatment of insomnia characterized by difficulty falling asleep (increased sleep latency). It has minimal potential for abuse, and no evidence of dependence or withdrawal effects has been observed, so ramelteon can be administered long term.
However, something better than melatonin for sleep, is the amino acid tryptophan, but take it by itself.
%i. Are there any di-substituted benzene drugs in meta positions?
It is relatively uncommon to find drugs with substituents in the meta position of a benzene ring, as the meta position is typically less favorable for the introduction of functional groups due to steric hindrance and electronic factors.
However, here is a list:
Ortho drugs:
Acetylsalicylic acid (Aspirin).
Meta drugs:
Phenylephrine (Sudafed PE).
Para drugs:
Paracetamol (Tylenol).
%i. What are some of the simplest prescription and non-prescription (over-the-counter) drugs?
Here is a list for drugs with low molecular weight (mw).
Prescription drugs:
Hydroxycarbamide, mw 76.
Non-prescription drugs:
Acetaminophen (Paracetamol), mw 151, a non-opioid analgesic.
Industrial chemistry.
%i. How can I separate oxygen from air?
3 methods:
-Cryogenic distillation. All these gases have different boiling points.
%i. Why do treatment plants use chlorine instead of hydrogen peroxide? When you add chlorine as a disinfectant, you have to add other chemicals to remove the chlorine. With hydrogen peroxide, it just decomposes to water and oxygen. Both kill bacteria and viruses.
So we have an answer, but not a direct answer. Most chemistry professors wouldn't know, since they don't work in the industry. Most government employees wouldn't know, because they've been doing this for possibly over a century. So the decision was made a long time ago. But 1 answer to look into, is economics.
70% hydrogen peroxide is about $1 to $2 a kilogram, and it appears 32-35% hydrogen peroxide is roughly the same price, even $1.50/kg. Liquefied chlorine goes for $.25 to $.35 per kilogram. This makes liquefied chlorine exponentially cheaper. It appears that 12% hydrogen peroxide, which can be bought at salon stores, is about the same price as liquefied chlorine. Perhaps reasoning suggests that 12% is not concentrated enough to use for drinking water treatment.
However, another thing to take into consideration is that chlorine remains in the water as a residual disinfectant (.2 to 2 ppm), whereas hydrogen peroxide does not. This helps protect the water from recontamination as it travels through the distribution system. It appears for Jardine plant in Chicago, their final check for chlorine concentration has to be .1 to .5 ppm, with a goal of .15 ppm.
An example of an octahedral Fe(II) complex is [Fe(H2O)6]2+ (hexaaquairon(II)).
Exenatide (Byetta).
Exenatide extended-release (Bydureon).
Liraglutide (Victoza).
Lixisenatide (Adlyxin).
Semaglutide injection (Ozempic).
Semaglutide tablets (Rybelsus).
Zepbound.
Ibuprofen (Advil, Motrin).
Fexofenadine (Allegra).
Amoxicillin, an antibacterial often prescribed by dentists.
Fomepizole, mw 82, used to treat methanol and ethylene glycol poisoning.
Metformin, mw 129, has a N=N. Used for type II diabetes, in 2020, it was the 3rd most commonly prescribed drug in the U.S.
Isoniazid, mw 137, an antibiotic for the treatment of tuberculosis. Has a N-N bond.
Hydralazine, mw 160, has a N-N bond.
Nitrofurantoin, mw 238, has a N=O resonance as well as N-N bond.
Pirbuterol, mw 240.
Menthol, mw 156, a k-opioid receptor agonist.
Phenylephrine, mw 167, used as a nasal decongestant.
Acetylsalicylic acid (Aspirin), mw 180.
Caffeine, mw 194.
Minoxidil, mw 209, used for baldness.
Diphenhydramine, mw 255.
-Using membranes. This is unfortunately for small-scale, does not surpass 40% oxygen purity, and used mainly for nitrogen gas.
-Using adsorbents. This does not surpass 95% oxygen purity.